CROSSLINKED HYALURONIC ACID IN DENTAL SURGERY
WHY USE HYALURONIC ACID IN DENTAL SURGERY ?
The gel of crosslinked hyaluronic acid (xHyA) is used for soft tissue recession surgery, guided tissue regeneration (GTR) as well as guided bone regeneration (GBR). The crosslinked hyaluronic acid-based gel, of non-animal origin, is optimized for regenerative peri-implant and periodontal applications.
- Accelerated tissue wound healing – Lower the post-operative inflammation process, reduce pain, and accelerate neoangiogenesis. [1,2]
- Improved outcome – Stabilizes coagulum and supports soft and hard tissue regeneration. [1-4]
- Improved predictability – Bacteriostatic action and reduced pathogen penetration for a lower risk of infection. [5]
EXPERTS USING CROSSLINKED HYALURONIC ACID GEL
SIX REASONS TO USE CROSSLINKED HYALURONIC ACID FOR A BETTER DENTAL SURGERY
- Greater outcome predictability – the crosslinked hyaluronic acid gel stabilizes the blood clot that attracts growth factors. It supports and accelerates bone (hard tissue) and soft tissue formation.[1-4, 17,19]
- Faster tissue healing – xHyA supports angiogenesis [1,10,19] for a better tissue vascularisation, and tissue formation [12,13,15-17] over an extended period. Its special formulation remains present throughout the various phases of the healing process due to its slow degradation pattern (several weeks).[17]
- Lower risks of infection – xHyA naturally has bacteriostatic properties that contribute to lowering the risk of infection.[5]
- Better patient experience – xHyA’s high molecular weight reduces swelling, pain, and discomfort after dental surgery during the healing process. [18,19]
- Better aesthetics – Support scar-less wound healing, especially important for dental surgery in the esthetic zone. [18,19]
- User friendly – For a predictable result, simply apply the gel directly to tissues (soft tissues, tooth root, bone) into the surgical site (in presence of blood).
Do not rinse. No risk of over-dosage. xHyA can be combined with other biomaterials such as:
bone substitutes like the Smartgraft as a „sticky bone“
collagen membranes like the Smartbrane, to extend their barrier effect over time by several weeks.
OUTSTANDING REGENERATIVE RESULTS OF PERIODONTAL TREATMENT WITH CROSSLINKED HYALURONIC ACID
HOW DOES HYALURONIC ACID WORK IN PERIODONTAL SURGERY ?
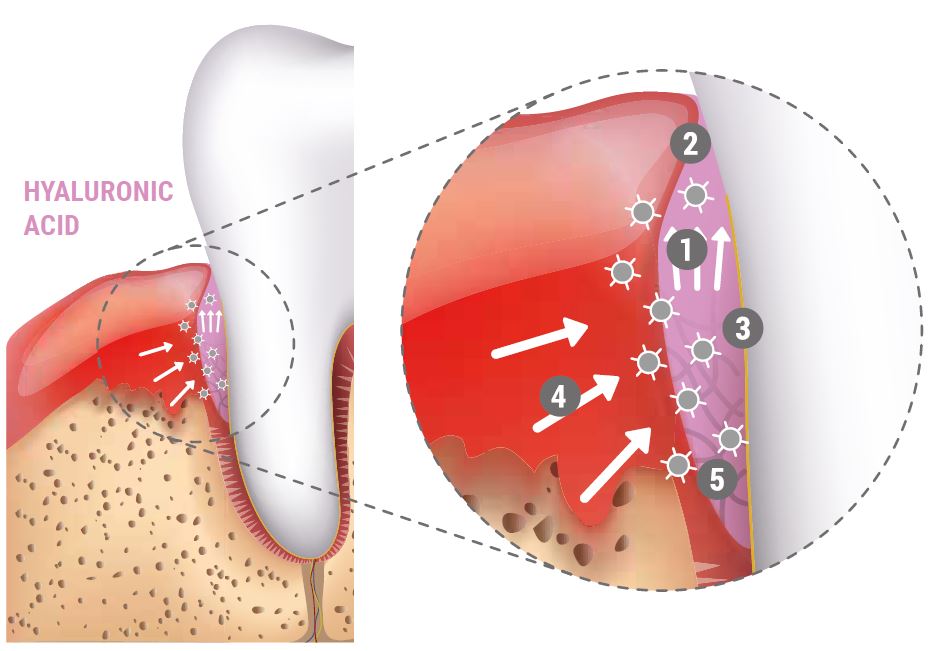
- Attracts blood
- Stabilizes coagulum and supports tissue regeneration
- Bacteriostatic effect provides protection
- Growth factors attracted by hyaluronic acid
- Coordinates inflammation and accelerates angiogenesis
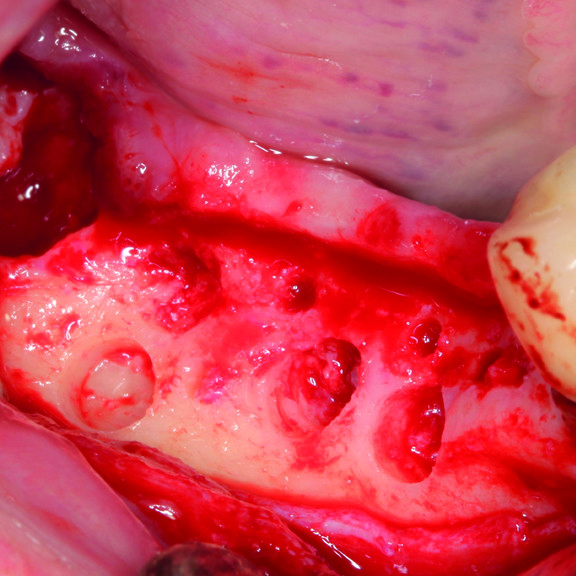
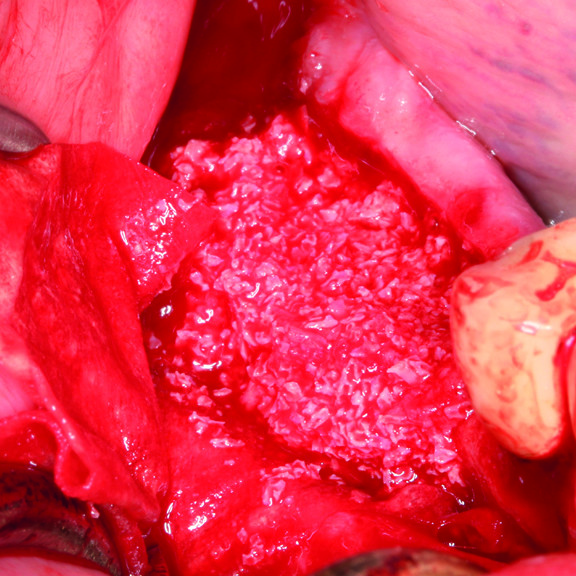
HOW TO USE CROSSLINKED HYALURONIC ACID GEL IN GBR ?
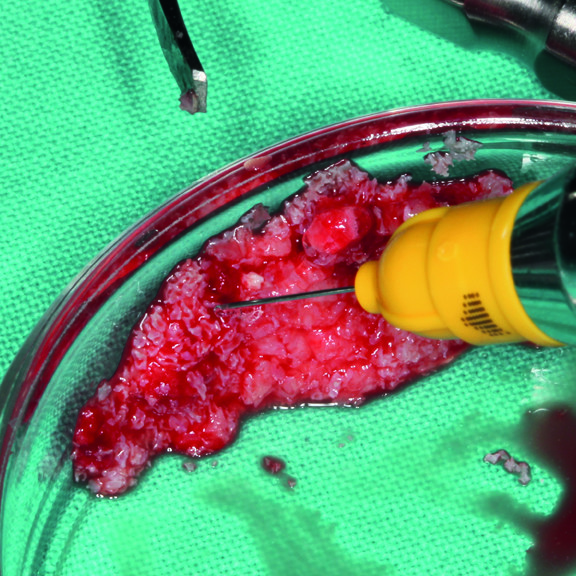
Sticky bone in 3 minutes with xHyA gel and Smartgraft.
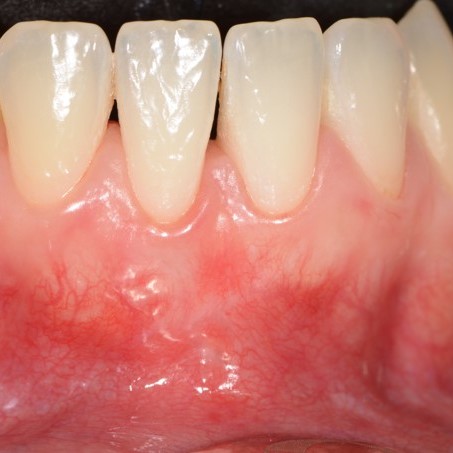
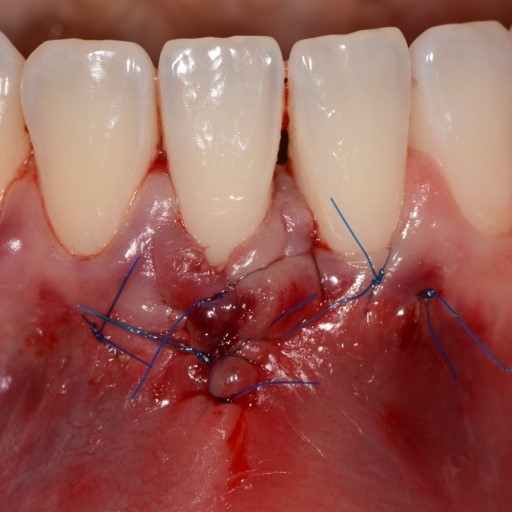
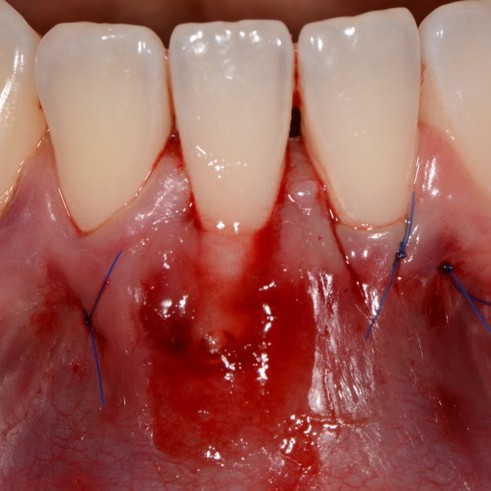
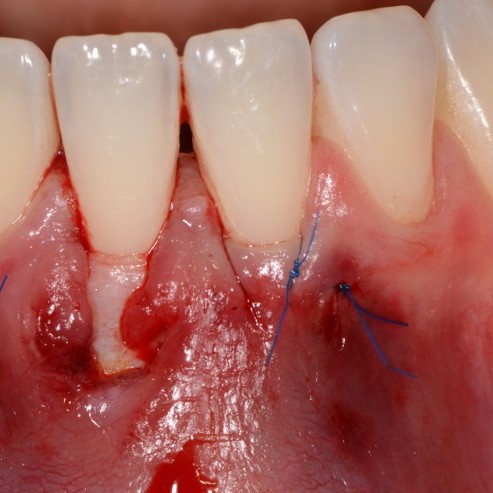
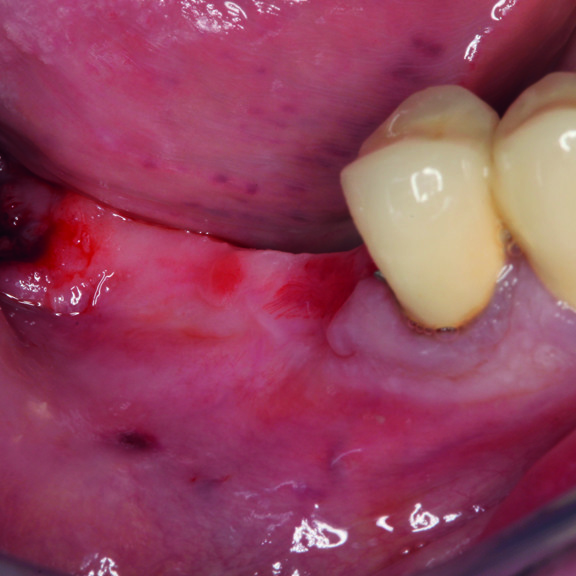
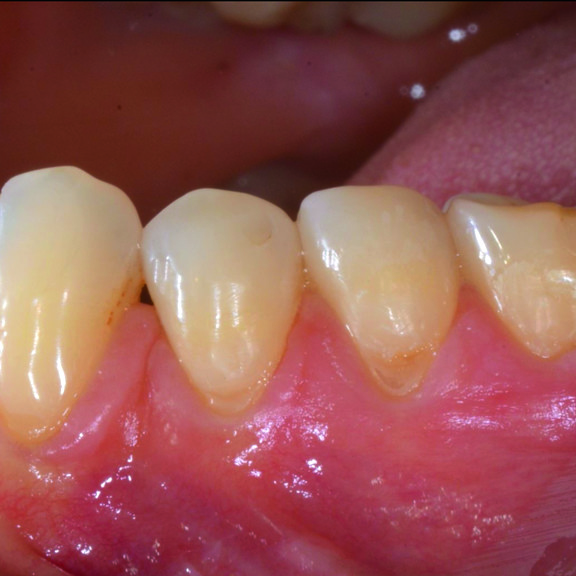
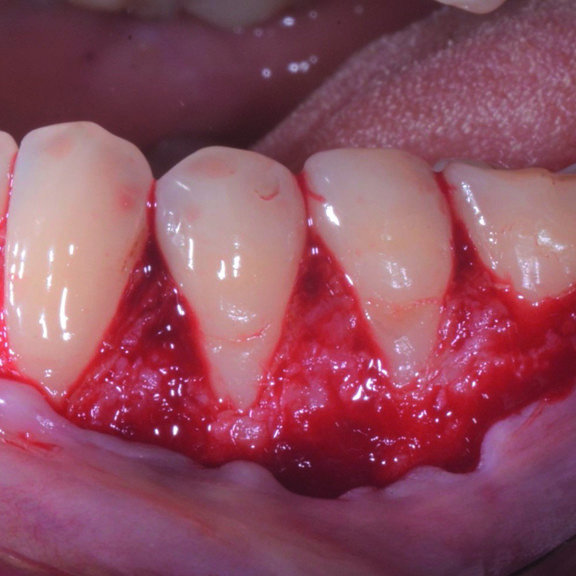
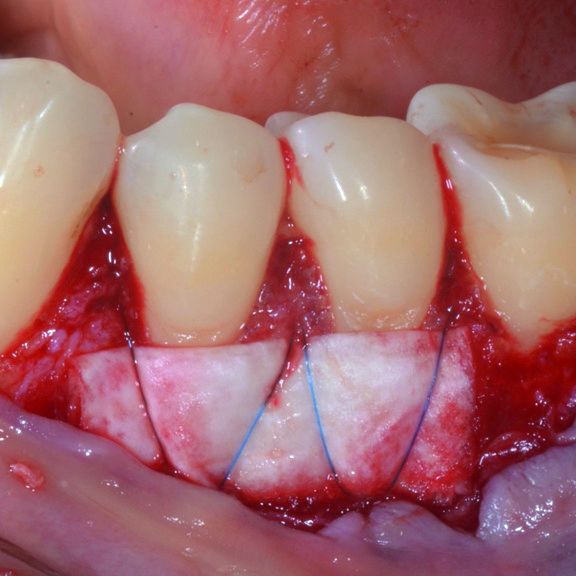
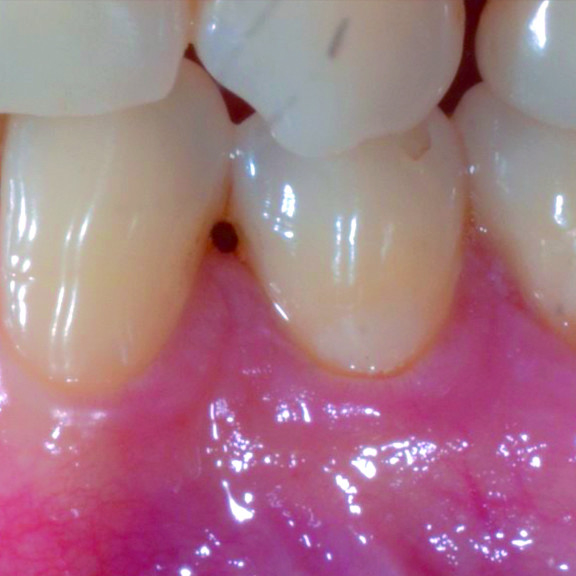
CASE REPORT : GINGIVAL RECESSION WITH CROSSLINKED HYALURONIC ACID
Deep Miller Class II gingival recession (Prof A Sculean) with the cross-linked hyaluronic acid gel
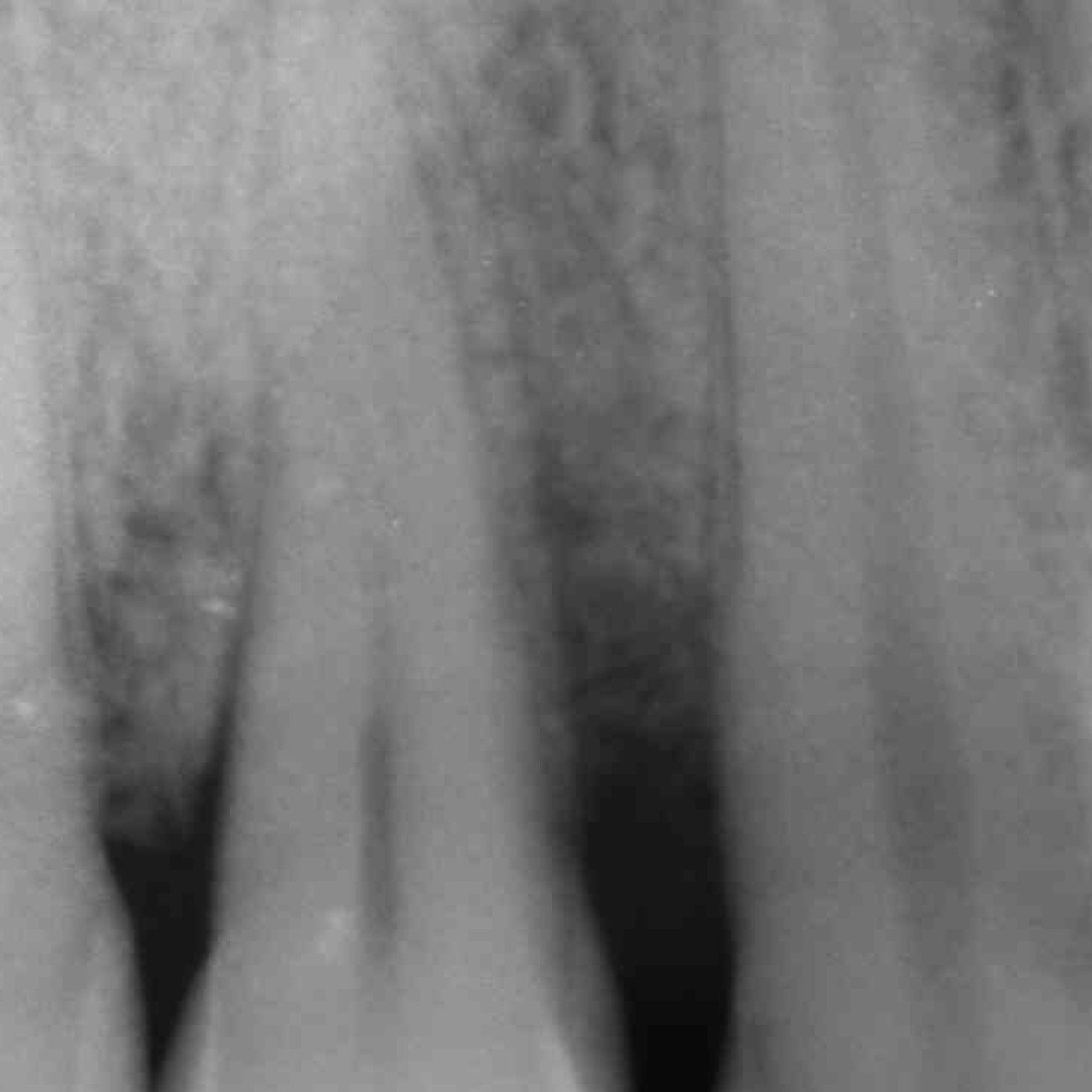
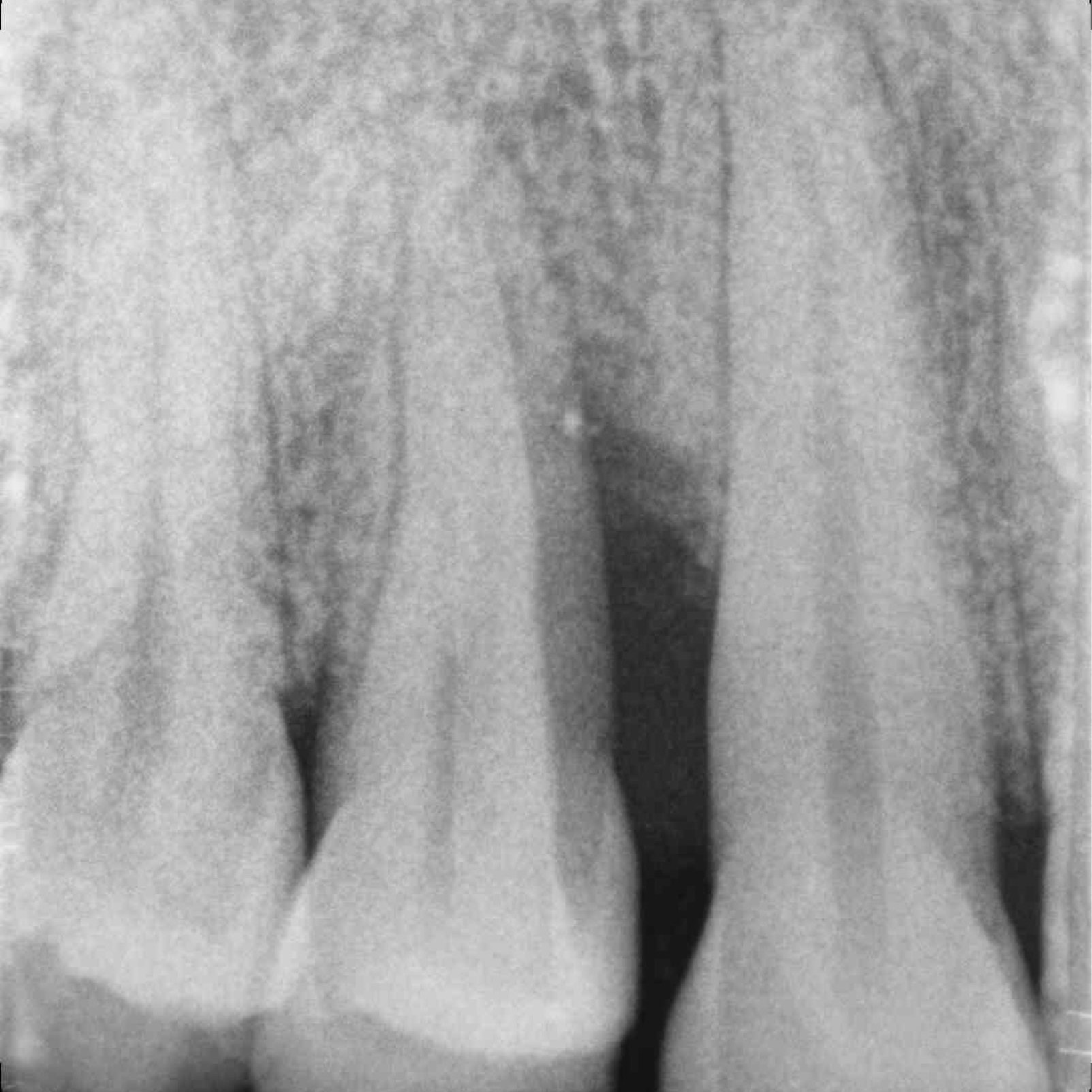
CASE REPORT : INFRABONY DEFECT WITH CROSSLINKED HYALURONIC ACID
Infrabony defect (Prof A Pilloni) with the cross-linked hyaluronic acid gel [20, 21]
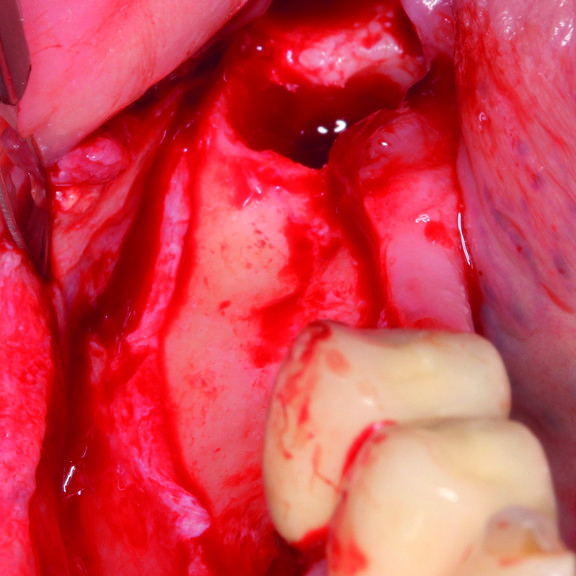
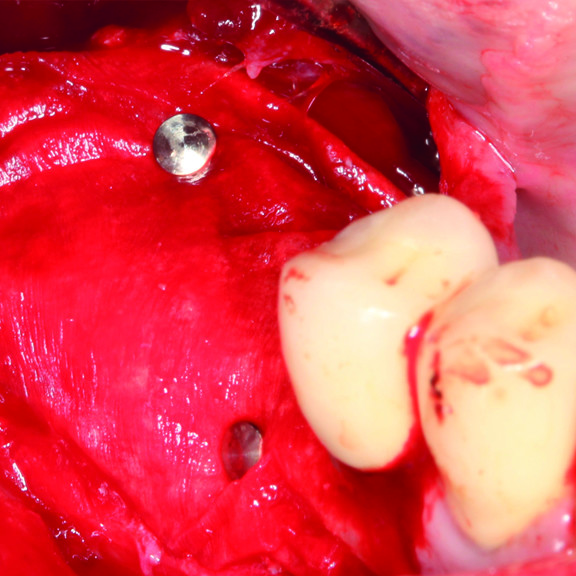
CASE REPORT: GBR WITH CROSSLINKED HYALURONIC ACID GEL
GBR prior to implant placement (Prof D Bozic) with the cross-linked hyaluronic acid gel and the Smartbrane
CASE REPORT : PERIODONTAL POCKET TREATMENT WITH CROSSLINKED HYALURONIC ACID
Pocket sealing after biofilm removal of a case affected by mucositis (Dr M Roncati)
CASE REPORT : SOFT TISSUE AUGMENTATION WITH CROSSLINKED HYALURONIC ACID
Soft tissue augmentation with cross-linked hyaluronic acid gel and Smartbrane (Dr T Pierchalla)
HOW DOES CROSSLINKED HYALURONIC ACID WORK ?
The cross-linked hyaluronic acid gel is designed specifically for application in the field of dental surgery. xHyA, as one of the main components of the extracellular matrix, is naturally present in the human body [6-8]. Studies have shown that the prolonged presence of xHyA during the healing process promotes healing by regeneration rather than reparation [9,10]. Besides accelerating the healing of soft tissue and bone[11-13], the bacteriostatic properties of xHyA also protect the wound [5].
Cross-linked hyaluronic acid remains present throughout the various phases of the healing process due to its slow degradation pattern (several weeks) [14]. In addition, it aids the surgical periodontal treatment after application to the root surface and soft tissue. This leads to faster wound closure, substantial pocket reduction, and enhanced attachment [1,3-4]. When mixed with bone substitute material, such as Smartgraft, it forms an easily manageable putty, which may, in addition, lead to accelerated bone formation [15,16, 32, 33] and reduced swelling and pain. [18,19] It has been also documented that high-molecular-weight cross-linked hyaluronic acid contributes to slowing down the resorption of collagen membranes, such as Smartbrane [30, 31].
SCIENTIFIC LITERATURE & CLINICAL STUDIES
Objective
To determine the effect of exogenous hyaluronic acid (HA) on healing of experimental wounds, responses in the hamster cheek pouch were measured after a hole was cut through the tissue with a biopsy punch.
Methodology
Fluorescence-labeled dextran was administered intravenously as a macromolecular tracer and the microcirculation was observed in vivo with a fluorescence microscope connected to a high-resolution television system. In one group a gelatin sponge soaked in 1.5 ml 16 mg/dl HA in water was applied topically at the time of injury and on postinjury days 1, 3, 5, and 7. The control group received the sponge soaked in the aqueous vehicle. Every 2 days after injury, the microcirculation was observed or histologic specimens were harvested. Wound size decreased almost twice as fast with HA compared with its vehicle (p less than 0.05). Healing was defined as time for total wound closure with at least one microvessel bridging the site of injury and required 16 or more days with vehicle but averaged less than 9 days with HA.
Results
Early during healing the repair site was surrounded by widespread extravasation of the fluorescent tracer, an index of inflammation; this area was reduced by two thirds 2 to 4 days after injury with HA compared with its vehicle (p less than 0.05). The density of perfused microvessels was twofold higher with HA 2 to 4 days after injury (p less than 0.05). However, microvessel density was similar in both groups by 6 days after injury and remained similar for at least 45 days after injury, which suggests that HA evoked no unusual angiogenic response. Histologic examination of fixed, stained specimens showed increases in intravascular leukocytes after injury and treatment-related differences in the distribution of intravascular leukocytes in 20 to 40 microns and 40 to 80 microns diameter microvessels 1 to 2 days after injury. Otherwise, leukocyte infiltration during healing was similar in both groups.
Conclusion
The mechanism for the beneficial action of HA on healing is unknown (at the time of this study: 1991). However, several in vitro studies suggest that HA is part of a feedback loop that promotes cell proliferation and migration in actively growing tissues. Alternatively, the role of HA in water homeostasis could favor tissue hydration, which has a well-known beneficial effect on healing.
Abstract
Wound healing involves a series of carefully modulated steps, from initial injury and blood clot to the final reconstituted tissue or scar. A dynamic reciprocity exists throughout between the wound, blood elements, extracellular matrix, and cells that participate in healing. Multiple cytokines and signal transduction pathways regulate these reactions. A major component throughout most of the process is hyaluronan, a straight-chain carbohydrate extracellular matrix polymer. Hyaluronan occurs in multiple forms, chain length being the only distinguishing characteristic between them. Levels of hyaluronan in its high-molecular-weight form are prominent in the earliest stages of wound repair. Progressively more fragmented forms occur in a manner not previously appreciated. We outline here steps in the wound healing cascade in which hyaluronan participates, as well as providing a review of its metabolism. Although described by necessity in a series of quantum steps, the healing process is constituted by a smooth continuum of overlapping reactions. The prevalence of hyaluronan in the wound (initially termed “hexosamine-containing mucopolysaccharide”), particularly in its early stages, was pointed out over half a century ago by the Harvard surgeon J. Engelbert Dunphy. It appears we are now returning to where we started.
Objective
Hyaluronic acid application has been proven to be beneficial in a number of medical disciplines. The aim of the current study was to clinically evaluate the effect of local application of hyaluronan gel in conjunction with periodontal surgery.
Methodology
Fourteen patients with chronic periodontitis having four interproximal intrabony defects (≥3 mm) with probing depth values >5 mm were included in this split-mouth study. Following initial nonsurgical periodontal therapy and re-evaluation, defects were randomly assigned to be treated with modified Widman flap (MWF) surgery in conjunction with either 0.8% hyaluronan gel (test) or placebo gel (control) application. Clinical attachment level (CAL), probing depth (PD), gingival recession (GR), plaque index (PI), and bleeding on probing (BOP) values were taken at baseline and 3 and 6 months. Differences between test and control sites were evaluated using a Wilcoxon signed-rank and a McNemar test. A Friedman and a Cochran test were used to test equal ranks over time.
Results
Statistically significant differences were noted for CAL and GR (P < 0.05) in favor of the test sites. No significant differences were found regarding PD, BOP, or PI values (P > 0.05). Hyaluronan gel application in conjunction with periodontal surgery appears to result in significant improvement of CAL and in a reduction in GR.
Conclusion
Hyaluronan gel application appears to improve the clinical outcome of MWF surgery.
Objective
This randomized clinical study examined the use of hyaluronic acid to treat infrabony periodontal defects over a period of 24 months.
Methodology
Forty subjects with a two-wall infrabony defect (probing depth [PD] >= 7 mm; clinical attachment level [CAL] >= 7 mm) were selected. The defects were randomly divided into two groups: sites treated with hyaluronic acid (test group) and those treated with open flap debridement (control group).
Results
The 12- and 24-month evaluations were based on clinical and radiographic parameters. The primary outcome variable was CAL. Test defects shows a mean CAL gain of 1.9 ± 1.8 mm, while the control defects yielded a significantly lower gain of 1.1 ± 0.7 mm. PD reduction was also significantly higher in the test group (1.6 ± 1.2 mm) than in the control group (0.8 ± 0.5 mm). Frequency distribution analysis of the study outcomes indicated that hyaluronic acid increased the predictability of clinically significant results (CAL gains >= 2 mm and PD reduction >= 2 mm) in the test group compared with the controls.
Conclusions
The treatment of infrabony defects with hyaluronic acid offered an additional benefit in terms of CAL gain, PD reduction, and predictability compared to treatment with open flap debridement.
Background
This investigation is one of a series of projects seeking to ascertain whether hyaluronic acid (HA) is therapeutically effective in tissue regeneration procedures. The rationale for these investigations is to test the hypothesis that HA can serve as a bioabsorbable carrier for other substrates as well as itself actively promote the regeneration of tissue.
Methods
In this paper, we report on the bacteriostatic and bactericidal properties of 3 molecular weight formulations of recombinant HA (low, 141 kD; medium, 757 kD; and high, 1,300 kD) on selected oral and non-oral microorganisms in the planktonic phase. Three concentrations of each HA formulation were screened, 0.5, 1.0, and 2.0 mg/ml, using a standard broth culture assay.
Results
Recombinant HA exerted varied bacteriostatic effects on all the bacterial strains tested depending on its molecular weight (MW) and concentration. The high concentrations of the medium MW HA had the greatest bacteriostatic effect, particularly on the Actinobacillus actinomycetemcomitans, Prevotella oris, Staphylococcus aureus, and Propionibacterium acnes strains. The 1.0 mg/ml concentration of high MW HA had the greatest overall bacteriostatic effect, inhibiting the growth of all 6 bacterial strains tested. Among the bacterial strains studied, HA was found to have no bactericidal effects, regardless of concentration or molecular weight.
Conclusions
The results of this study suggest that HA in the MW range of 1,300 kD may prove beneficial in minimizing bacterial contamination of surgical wounds when used in guided tissue regeneration surgery.
Abstract
Hyaluronan has been implicated in biological processes such as cell adhesion, migration and proliferation. Traditionally, it was thought to be associated with the extracellular matrix, but, hyaluronan may also have unimagined roles inside the cell. Investigation of hyaluronan synthesis and degradation, the identification of new receptors and binding proteins, and the elucidation of hyaluronan-dependent signaling pathways are providing novel insights into the true biological functions of this fascinating molecule.
Abstract
Accumulation and turnover of extracellular matrix components are the hallmarks of tissue injury. Fragmented hyaluronan stimulates the expression of inflammatory genes by a variety of immune cells at the injury site. Hyaluronan binds to a number of cell surface proteins on various cell types. Hyaluronan fragments signal through both Toll-like receptor (TLR) 4 and TLR2 as well as CD44 to stimulate inflammatory genes in inflammatory cells. Hyaluronan is also present on the cell surface of epithelial cells and provides protection against tissue damage from the environment by interacting with TLR2 and TLR4. Hyaluronan and hyaluronan-binding proteins regulate inflammation, tissue injury, and repair through regulating inflammatory cell recruitment, release of inflammatory cytokines, and cell migration. This review focuses on the role of hyaluronan as an immune regulator in human diseases.
Abstract
Midgestation fetal wound healing is characterized by healing without fibrosis or scar formation. The mechanisms that underlie this remarkable process are mediated in part through a fetal wound extracellular matrix rich in hyaluronic acid. In this study a newly developed assay was used to determine the hyaluronic acid levels in fetal and adult wound fluid. Adult wound fluid had a rapid increase in hyaluronic acid, which peaked at 3 days and decreased to 0 by 7 days. In contrast levels of hyaluronic acid in fetal wound fluid increased rapidly and remained significantly elevated for 3 weeks. This prolonged presence of hyaluronic acid in the matrix of fetal wounds creates a ‘permissive’ wound environment that promotes fetal fibroblast movement and proliferation and inhibits cytodifferentiation. Such a matrix environment promotes healing by regeneration rather than by scarring. This observation has therapeutic implications. The prolonged application of hyaluronic acid or hyaluronate protein complexes to wounds in children or adults may modulate healing in a manner that makes the wounds more fetal-like.
Background
Fetal wound healing is characterized by minimal inflammation, mild fibroplasia and rapid, but organized collagen deposition such that scarring is not apparent. The matrices of fetal wounds differ greatly from adult wounds in that fetal wounds are persistently enriched with hyaluronic acid (HA). It has been shown that a reduction in fetal rabbit wound HA results in an adult-like healing response with increased fibroplasia and neovascularization. These observations suggest that HA can modulate cellular activity in fetal repair.
Methodology
Therefore, this study was designed to define the effect of HA on fetal fibroblast function. Fibroblasts from the skin of fetal rabbits were isolated and maintained in culture medium containing either no HA (controls), 1 microgram/ml, 10 micrograms/ml or 100 micrograms/ml of HA (n = 6 for each group). Fibroblast proliferation was quantitated by DNA content in each culture, and collagen and noncollagen protein synthesis were analyzed by incorporation of [3H] proline into collagenase-digestible and collagenase-nondigestible protein, respectively.
Results
At all concentrations tested, HA significantly inhibited fetal fibroblast proliferation (p < 0.02), but stimulated collagen (p < 0.002) and noncollagen protein (p < 0.005) synthesis. These findings provide further evidence that HA affects the function of fetal fibroblasts. Moreover, this study in conjunction with previous in utero findings suggests that HA may have a regulatory influence in scarless fetal healing by affecting cellular function during the repair process.
Abstract
To meet the growing need for tissue replacement materials for our aging population, the development of new adaptive biomaterials is essential. The tissues with the highest demand for implant materials are skin and bone. These tissues share various similarities, including signaling pathways and extracellular matrix composition. Glycosaminoglycans such as hyaluronan and chondroitin sulfate are the major organic extracellular matrix components. They modulate the attraction of skin and bone precursor cells and their subsequent differentiation and gene expression and regulate the action of proteins essential to bone and skin regeneration. The precise action of glycosaminoglycans varies according to their structural composition mainly in respect to the degree of sulfation and polymer length. Changes in the glycosaminoglycan composition are frequently seen in physiological and pathological remodeling processes, such as bone formation or scaring. Here, we review the current state of knowledge of how the most common glycosaminoglycan, chondroitin sulfate and hyaluronan, interact with bone and skin cells, and summarize their potential in tissue engineering for skeletal and skin diseases.
Objective
Hyaluronic acid (HA) is one of the essential components of extracellular matrix, which plays a predominant role in tissue morphogenesis, cell migration, differentiation, and adhesion. Bone allografts are frequently used to repair and reconstruct bone defects.
Methodology
In this study, two cavities of 3 mm diameter and depth have been created in the right tibia of 30 mature rabbits in accordance with the principles of general surgery. One of the cavities in the tibia is filled with HA and bovine bone graft and the other is filled with only spongiosal bone graft, for the purpose of control. On the 20th, 30th, and 40th days, rabbits have been sacrificed in equal numbers and defective regions have been extracted. The Kruskal–Wallis test was applied to the data obtained in the result of histopathologic survey of specimens.
Conclusion
The cavities that have been filled with HA and bone graft have shown higher scores than the control group during every period of the study.
Background
To study the osteoinductive action of hyaluronic acid (HA), we examined the effects of applying an elastoviscous high-molecular HA preparation on bone wound healing after bone marrow ablation.
Methodology
The middiaphyses of cortical bones from rat femurs were perforated with a round bar, and excavated marrow cavities were filled immediately with high-molecular HA. Bone marrow ablation without HA was used to prepare controls. On post-ablation days 1, 2, 4, 7, and 14, animals were perfusion-fixed with an aldehyde mixture, and dissected femurs were examined by means of light, transmission-, and scanning-electron microscopy.
Results
In controls, the wounded marrow cavities were first filled with blood and fibrin clots (days 1 and 2), then with granulated tissues containing macrophages, neutrophils, and fibroblastic cells (day 4). New bone formation by differentiated osteoblasts was observed at 1 week post-ablation; at 2 weeks, the perforated cortical bones and marrow cavities were filled mostly with newly formed trabecular bone. In bones to which HA had been applied, new bone formation already had been induced by day 4 on both the peri- and endosteal surfaces of the existing cortical bones. At 1 week post-ablation, marrow cavities were completely filled with newly formed trabecular bones, in which active bone remodeling by osteoblasts and osteoclasts had occurred. Granulated tissues were replaced rapidly by normal marrow cells.
Conclusion
These results suggest that high-molecular HA is capable of accelerating new bone formation through mesenchymal cell differentiation in bone wounds.
Background
Cosmetic procedures are growing ever more common, and the use of soft tissue fillers is increasing. Practicing physicians need to be aware of the biological behavior of these products in tissue to enable them to respond to any safety concerns that their patients raise.
Objectives
To provide an overview of the metabolism of 1,4-butanediol diglycidyl ether (BDDE)-crosslinked hyaluronic acid (HA) dermal fillers and to examine the safety of the resulting byproducts.
Methods
A review of available evidence was conducted.
Results
After reaction with HA, the epoxide groups of BDDE are neutralized, and only trace amounts of unreacted BDDE remain in the product (<2 parts per million). When crosslinked HA, uncrosslinked HA, and unreacted BDDE degrade, they break down into harmless byproducts or into byproducts that are identical to substances already found in the skin.
Conclusion
Clinical and biocompatibility data from longer than 15 years support the favorable clinical safety profile of BDDE-crosslinked HA and its degradation products. Given the strength of the empirical evidence, physicians should be confident in offering these products to their patients.
Objective
Sinus floor augmentation (SFA) using bone grafting materials, and in particular calcium phosphates (CaP), is a well-established pre-implantology procedure. The use of CaP simplifies SFA procedures. β-tricalcium phosphate (β-TCP) is amply used for SFA. This study evaluated the clinical and osteogenic performance of β-TCP granules (TCP-G) and a β-TCP putty (TCP-P) bone graft material. TCP-P consisted of TCP-G in a hyaluronic acid (HyA) carrier. Bone formation, volume stability and osteogenic marker expression after bilateral SFA in patients was assessed.
Methodology
Eight patients were selected for a split-mouth design. Biopsies obtained six months after SFA, were processed for immunohistochemical analysis of collagen type I (Col I), alkaline phosphatase (ALP), osteocalcin (OC) and bone sialoprotein (BSP). Histomorphometric analysis determined bone, grafting material and marrow space percentages. Cone-beam computed tomography was used to calculate the graft volume and its stability.
Conclusion
Both materials allowed excellent bone regeneration and volume stability. TCP-P displayed better surgical handling properties, greater bone formation, higher expression of Col I, ALP, OC and BSP; as well as significantly lower grafting volume reduction values. HyA had no adverse effect on TCP-P performance. Due to its clinical and osteogenic performance, TCP-P can be regarded as excellent bone grafting material for SFA.
Objective
In this study we evaluated the effects of sodium hyaluronate (HY) in the healing process of tooth sockets of rats.
Methodology
Immediately after the extraction of the upper first molars of male Holtzman rats, right sockets were treated with 1% HY gel (0.1 ml), while left sockets were used as control (blood clot). The animals were sacrificed at 2, 7, and 21 days after tooth extraction and upper maxillaries processed for histological and morphometric analysis of the apical and medium thirds of the sockets. Carbopol, an inert gel, was used to evaluate the mechanical effect of gel injection into sockets. Expression of bone morphogenetic protein-2 (BMP-2) and osteopontin (OPN) was determined by immunohistochemistry at 1, 2, 3, 4, 5, and 7 days after tooth extraction.
Results
Histological analysis showed that HY treatment induced earlier trabecular bone deposition resulting in a bone matrix more organized at 7 and 21 days after tooth extraction. Also, HY elicited significant increase in the amount of bone trabeculaes at 7 and 21 days after tooth extraction (percentage of trabecular bone area at 7 days: 13.21″ 4.66% vs. 2.58″ 1.36% in the apical third of control sockets) and in the vessels counting at 7 days. Conversely, the number of cell nuclei was decreased in HY-treated sockets. Additionally, expression of BMP- 2 and OPN was enhanced in HY-treated sockets compared with control sockets.
Conclusion
These findings suggest that HY accelerates the healing process in tooth sockets of rats stimulating the expression of osteogenic proteins.
Objectives
Histologically evaluate the effects of cross-linked HA alone or combined with a collagen matrix (CM = Fibro Gide) on the periodontal wound healing/regeneration in intrabony defects.
Material and methods
Two-wall intrabony defects (5 mm wide, 5 mm deep) were surgically created at the distal and mesial aspects of mandibular premolars in six beagle dogs. The 24 defects were randomly treated as follows: open flap debridement (OFD) + HA, OFD +CM, OFD + HA+ CM (HA/CM) and OFD alone (control). At 2 months, the animals were euthanized for histological evaluation.
Results
The HA (2.43±1.25 mm) and HA/CM (2.60±0.99 mm) groups yielded statistically significantly ( P < 0.05) greater formation of new attachment (i.e., linear length of NC adjacent to newly formed bone, with inserting collagen fibers) compared with OFD (0.55±0.99 mm) group. Among the 4 treatment groups, the HA/CM group demonstrated the highest amount of regenerated tissues, although no statistically significant differences in any of the histometric parameters were observed between the HA and HA/CM groups.
Within their limits, it can be concluded that cross-linked HA alone or combined with CM
promotes periodontal wound healing/ regeneration in two-wall intrabony defects in dogs.
Conclusion
The present data have for the first time provided histologic evidence for periodontal regeneration of gingival recession defects following treatment with CAF and HA.
Objectives
This study aims to evaluate the effects of two different concentrations of topical hyaluronic acid (HA) on postoperative patient discomfort and wound healing of palatal donor sites after free gingival graft (FGG) surgery.
Methodology
Thirty-six patients requiring FGG were randomly assigned into three groups in an examiner-masked, randomized, controlled clinical trial. After harvesting palatal grafts, 0.2% and 0.8% HA gels were used in test groups 1 and 2, respectively. Gels were applied on donor sites and protected with periodontal dressing in the test groups, whereas the wound was covered only with periodontal dressing in the control group. On days 3, 7, 14, and 21, pain and burning sensation were recorded using a visual analog scale (VAS) as well as other parameters such as complete epithelization (CE) and color match on days 3, 7, 14, 21, and 42.
Results
Test groups experienced less pain than the control group on days 3 and 7 (P <0.001 and P <0.001, respectively). Mean VAS score for burning sensation was higher in the control group on day 3 compared with test groups 1 and 2 (P = 0.03 and P = 0.02, respectively). CE in all patients was achieved on day 21 in both test groups, whereas it was achieved on day 42 in the control group. The test groups showed higher color match scores than the control group on days 21 (P <0.001 and P <0.001, respectively) and 42 (P = 0.004 and P = 0.002, respectively).
Conclusion
Topical application of HA exhibits positive impact on postoperative pain and burning sensation, and accelerates palatal wound healing in terms of epithelization and color match.
Objectives
A cemental tear (CeT) is a special type of surface root fracture that may cause periodontal and even periapical tissue destruction. Unfortunately, there is limited knowledge as to how these rare cases can effectively be treated. The present case is the first reported in the literature treating a bony defect caused by a cemental tear with hyaluronic acid (HA) and a collagen membrane. The aim of this case report is to present a regenerative surgical approach with clinical and tomographic success and stability at 2-year follow-up.
Case Presentation
A 61-year-old patient presented with spontaneous pain and gingival swelling over his right central maxillary incisor. Radiographically, a radiolucent area was observed in the medial third between both central incisors. The tomographic evaluation showed a buccal bone dehiscence and a bony defect. Once the differential diagnosis with an endodontic-periodontal lesion and root fracture was performed, CeT was the presumptive diagnosis. During the exploratory flap surgery, a small root fragment (CeT) on the mesial side of the tooth was founded and removed. The bony lesion was treated with hyaluronic acid (HA) and a resorbable collagen membrane. At 2-year follow-up clinical, radiographic, and tomographic success was observed.
Conclusion
A CeT-associated bony defect could be successfully treated after removing cemental fragments and performing a regenerative approach using HA and a resorbable collagen membrane.
Background
This study evaluates the clinical outcomes of a novel approach in treating deep intrabony defects utilizing papilla preservation techniques with a combination of hyaluronic acid (xHyA – Hyadent BG) and deproteinized porcine bone mineral.
Clinical Procedure
23 patients with 27 intrabony defects were treated with a combination of hyaluronic acid (Hyadent BG) and deproteinized porcine bone mineral. Clinical attachment level (CAL), pocket probing depth (PPD), gingival recession (REC) were recorded at baseline and 6 months after the surgery.
Outcomes
At 6 months, there was a significant CAL gain of 3.65 ± 1.67 mm (p < 0.001) with a PPD reduction of 4.54 ± 1.65 mm (p < 0.001), which was associated with an increase in gingival recession (0.89 ± 0.59 mm, p < 0.001). The percentage of pocket resolution based on a PPD ≤4 mm was 92.6% and the failure rate based on a PPD of 5 mm was 7.4%.
Conclusion
The present findings indicate that applying a combined cross-linked hyaluronic acid (Hyadent BG) and xenograft approach in deep intrabony defects provides clinically relevant CAL gains and PPD reductions compared to baseline values and is a valid new approach in treating intrabony defects.
Objectives
Enamel matrix derivative (EMD) in combination with flap designs aiming to maximally preserve the interdental soft tissues, is still considered the gold standard in the regenerative treatment of periodontal intrabony defects. However, increasing evidence from preclinical and clinical studies indicates that cross-linked hyaluronic acid (xHyA – Hyadent BG) possesses a number of positive biologic effects on periodontal wound healing and regeneration. However, at present, there are virtually no data from clinical studies evaluating the effects of xHyA when used in conjunction with reconstructive periodontal surgery as compared to the use of EMD. Therefore, the aim of this randomized controlled clinical trial was to compare the clinical outcomes obtained in intrabony defects following regenerative periodontal surgery using the single flap approach (SFA) in conjunction with either cross-linked hyaluronic acid or EMD.
Methodology
Thirty-two intrabony defects in 32 healthy subjects were randomly assigned: xHyA (test group) or EMD (control group). Clinical attachment level (CAL), probing depth (PD), gingival recession (REC) and bleeding on probing (BOP) were recorded at baseline,12-,18-and 24-months after surgery.
Results
At 24-months, both treatments resulted in statistically significant clinical improvements evidenced by PD-reduction and CAL-gain. The mean CAL-gain was 2.19 ± 1.11 mm in the test and 2.94 ± 1.12 mm in the control sites, respectively, without statistically significant difference between the groups. PD-reduction was statistically significantly higher for the control group (4.5 ± 0.97 mm) than the test group (3.31 ± 0.70 mm). Test sites showed slightly lower REC values (1.19 ± 0.75 mm) than the control sites (1.69 ± 0.70 mm). No statistically significant changes were observed in terms of BOP changes within and between the groups.
Conclusions
Within their limits the present findings indicate that a) both treatments led to statistically significant long-term clinical improvements, and b) cross-linked hyaluronic acid (xHyA) appears to represent a valuable alternative for regenerative treatment in intrabony periodontal defects.
Objectives
To clinically and histologically evaluate in dogs the healing of gingival recessions treated with coronally advanced flap (CAF) with or without cross-linked hyaluronic acid (xHyA – Hyadent BG).
Material and methods
Gingival recession defects were surgically created on the vestibular side of both maxillary canines in 8 dogs. After 8 weeks of plaque accumulation, the 16 chronic defects were randomly treated with either CAF alone or CAF and cross-linked hyaluronic acid-gel (CAF/xHyA). Clinical and histological outcomes were evaluated at 10 weeks post-surgically.
Results
Compared to baseline, the clinical measurements at 10 weeks revealed a statistically significant decrease in gingival recession for both CAF (p<0.01) and CAF/xHyA (p<0.001) groups. Statistically significant differences were found in clinical attachment level (p<0.05) and width of gingival recession (p<0.01) favoring the CAF/xHyA group. Bone formation was statistically significantly greater in the CAF/xHyA group than in the CAF group (1.84±1.16mm vs., 0.72±0.62mm respectively, P<0.05). Formation of cementum and connective tissue attachment were statistically significantly higher in the CAF/xHyA group compared with the CAF group (i.e. 4.31±1.78mm versus 2.40±1.35mm and 1.69±0.98mm versus 0.74±0.68mm, respectively (P<0.05)).
Conclusion
The present data have for the first time provided histologic evidence for periodontal regeneration of gingival recession defects following treatment with CAF and cross-linked hyaluronic acid (xHyA).
Objective
To clinically evaluate the healing of mandibular Miller Class I and II isolated gingival recessions treated with the modified coronally advanced tunnel (MCAT) or laterally closed tunnel (LCT) combined with cross-linked hyaluronic acid (xHyA) and subepithelial connective tissue graft (SCTG).
Methodology
Twelve healthy patients exhibiting one isolated mandibular Miller Class I or II (Cairo Class 1) gingival recession of a depth of ≥ 3 mm, were consecutively treated with the MCAT or LCT in conjunction with HA and SCTG. Treatment outcomes were assessed at baseline and at least 6 months postoperatively. The primary outcome variable was complete root coverage (CRC).
Results
Postoperative pain and discomfort were low and no complications such as postoperative bleeding, allergic reactions, abscesses, or loss of SCTG occurred. After a mean follow-up of 18.9 ± 10 months, statistically significant (P < .0001) root coverage was obtained in all 12 defects. CRC was measured in six out of the 12 cases (50%), four cases showed a root coverage of over 95%, while the remaining two cases reached 80% and 85%. Mean root coverage was 96.09%. Mean keratinized tissue width increased from 1.6 ± 0.8 mm to 4.9 ± 1.3 mm (P < .0001) from baseline to follow-up, while mean probing depth showed no statistically significant changes (1.8 ± 0.9 mm vs 1.3 ± 0.5 mm).
Conclusion
Within their limits, the present results indicate that the described treatment approach may lead to predictable root coverage of isolated mandibular Miller Class I and II (Cairo Class 1) gingival recessions.
Objectives
To evaluate the healing of multiple adjacent type 1 and 2 gingival recessions (RT1 and RT2) treated with the modified coronally advanced tunnel (MCAT) or the laterally closed tunnel (LCT) in conjunction with a cross-linked hyaluronic acid (xHyA) and subepithelial palatal connective tissue grafts.
Method and materials
Fifteen healthy patients exhibiting multiple adjacent mandibular or maxillary RT1 and RT2 of a depth of ≥ 2 mm, were treated with the MCAT or LCT in conjunction with cross-linked hyaluronic acid and subepithelial palatal connective tissue grafts. Results were assessed at baseline and after a minimum of 6 months. The primary outcome variable was root coverage. Esthetic outcomes were evaluated on photographs using the root coverage esthetic score.
Results
Postoperative pain and discomfort were low and no complications occurred. Data analyses were performed at patient level. After a mean follow-up of 17 ± 5.4 months, statistically significant root coverage was obtained in all 15 cases (P < .0001). Complete root coverage was obtained in 3 out of 15 cases (20%). Root coverage amounted to > 95% in three patients, was between 90% and 95% in four patients, and reached 87.5% in another patient. In three further patients root coverage measured 75%, 77%, and 64.6%, respectively. Mean root coverage measured 85.1 ± 23.2%. Mean keratinized tissue width increased from 2.5 ± 1.0 mm to 3.7 ± 0.7 mm (P < .0001) from baseline to follow-up, while mean probing depth showed no statistically significant changes (1.3 ± 0.5 mm vs 1.5 ± 0.5 mm). The mean root coverage esthetic score was 7.9 ± 1.9, while in the three cases exhibiting complete root coverage, a maximum root coverage esthetic score (10) was given for all treated teeth.
Conclusion
Within their limits, the present results indicate that the described treatment approach may lead to predictable root coverage of multiple mandibular and maxillary RT1 and RT2.
Objectives
The aim of the study was to evaluate the effectiveness of cross-linked Hyaluronic Acid (xHyA) gel on facial swelling, pain, and truisms after extraction of impacted mandibular third molars.
Methodology
This randomized, double-blinded, splitmouth clinical trial included 14 patients. For each patient, a combination of cross-linked HA with Gelfoam scaffold were randomly applied to one extraction site, while Gelfoam alone was applied to the other extraction site. Measurements of three facial reference points, pain and maximum mouth opening were recorded preoperatively, as well as on the 2nd, 4th and 7th days after the surgery.
Results
The scores for facial swelling, pain and trismus were the highest on the 2nd postoperative day and decreased gradually on the 4th to the 7th days in both groups. Cross-linked HA group demonstrated statistically significant reduction in swelling, pain, and trismus on the 7th postoperative day when compared to its control group (p<0.05).
Conclusion
The application of cross-linked HA after extraction of impacted mandibular third molars has a positive impact on postoperative swelling, pain and trismus after the extraction of impacted lower third molars.
Introduction
Biphasic calcium phosphate (BCP) is very widely used as a grafting material around dental implants. The properties of such material can be enhanced by adding interpositional graft materials to enhance osteoinduction. Hyaluronic acid (HyA) is an example of osteopromoting materials that can be added to the BCP to enhance its osteoinductive properties.
Objectives
Histological evaluation of using HyA with BCP on bone healing around dental implants.
Methodology
This study was a split mouth design. It was conducted on 9 mongrel dogs. The dogs were allocated into two groups: Group A (Study Group): The right side of the mandible received dental implants with biphasic calcium phosphate bone graft mixed with hyaluronic acid following extraction of the mandibular third premolar. Group B (Control Group): The left side of the mandible received dental implants with biphasic calcium phosphate bone graft only following extraction of the mandibular third premolar. Dogs were sacrificed at 2, 4 and 6 weeks postoperatively. Segments containing the implant and bone graft were retrieved with adjacent bone to be prepared for histological examination using Haematoxylin and eosin stain and Trichrome stain.
Results
All animals survived well, and remained active and alert all over the course of the experiment. Both groups were characterized by new bone formation. The newly formed bone was more evident in association with group (A).
Conclusion
HyA accelerates the onset of new bone formation when combined with BCP for bone augmentation in the treatment of osseous defects.
Objective
Alveolar atrophy following tooth extraction remains a challenge for future dental implant placement. Immediate implant placement and postextraction alveolar preservation are two methods that are used to prevent significant postextraction bone loss. The purpose of this study is to investigate the usefulness of hydroxyapatite / beta tricalcium phosphate (HA/BTCP) with hyaluronic acid (HyA) for alveolar sockets preservation.
Methodology
Thirty-two New Zealand white rabbits were subjected to the lower left incisor extraction. The rabbits were equally divided into three groups. The extracted sockets (n = 12/group) were filled with: HA/BTCP, HA/BTCP + HyA and blood clot (control). All rabbits were sacrificed for histological and histomorphometric evaluation after 4- and 8-week healing periods. .
Results
The results demonstrated that all sites examined in this study displayed evidence of new bone formation. A statistically significant difference in the amount of new bone formation were found between sites that healed for an average of 8 only. The results demonstrating approximately 78%,68 % and 63% of new vital bone formation for groups grafted with HA/BTCP +HyA , HA/BTCP and control group respectively after 8 weeks postoperatively.
Conclusion
In conclusion these results exhibited that the use of hydroxyapatite / beta tricalcium phosphate with hyaluronic acid appears to be more efficient in osteoconduction when compared with of hydroxyapatite / beta tricalcium phosphate alone and could be promising strategy for preservation of alveolar sockets.
Objective
This study evaluated the effects of hyaluronic acid (HyA) on bone repair of human dental sockets.
Methodology
Thirty-two lower first premolars were extracted from 16 patients (2 per patient) for orthodontic reasons. Following the extractions, one socket was randomly filled with 1% HyA gel, while the other was allowed to naturally fill with blood clot. After 30 and 90 days of surgery, patients underwent cone beam computed tomography. Five central orthoradial slices were captured from each socket. The gray intensity was measured in each image and results were reported as mean percentage of bone formation. The buccolingual alveolar ridge width was measured and dimensional changes were compared between the postoperative int ervals. The pattern of alveolar trabecular bone was evaluated through the fractal dimension.
Results
Treated sockets showed a higher percentage of bone formation and fractal dimension values (58.17% and 1.098, respectively) compared with controls (48.97% and 1.074, respectively) in the 30-day postoperative period (p < 0.05). After 90 days, there was no significant difference between groups. Additionally, no significant difference was found between groups regarding the alveolar dimensions (p > 0.05).
Conclusion
Use of 1% hyaluronic acid gel after tooth extraction accelerates bone repair in human dental sockets.
Background
Previous studies on ridge preservation focusing on fresh extraction sockets using graft materials for ridge preservation procedures have reported a delay in the tissue modelling and remodelling phases. The objective of this study is to evaluate the effect of hyaluronic acid (HyA) on healing of infected sockets.
Methodology
Six beagle dogs were used in this study. Both mandibular third premolars were hemisected, and the distal roots were extracted. Subsequently, periodontal and endodontic lesions were induced at the remaining mesial root. After communication of the periodontal lesion, an endodontic periapical lesion was observed at 4 months, and the mesial roots of both the right and left sides were extracted. HyA was applied into the socket of the test group, and no treatment was administered to the other group (control group). Three months after extraction of the mesial roots, the dogs were sacrificed, and histologic evaluations were performed.
Results
The sockets were filled by mineralized bone (47.80% ± 6.60%) and bone marrow (50.47% ± 6.38%) in the control group, whereas corresponding values were 63.29% ± 9.78% and 34.73% ± 8.97% for the test group, respectively. There was a statistically significant difference between the groups. Reversal lines and a copious lineup of osteoblasts were observed in the middle and apical parts of the sockets in the test group.
Conclusion
An infected socket shows delayed healing of the socket wound, and hyaluronic acid (HyA), because of its osteoinductive, bacteriostatic, and anti-inflammatory properties, may improve bone formation and accelerate wound healing in infected sockets.
Objective
To examine the in vitro biokinetics of hyaluronic acid (HA) from a collagen membrane (CM) and to evaluate the in vivo effect of immersion of the CM in HA solution on its degradation in streptozotocin (STZ)‐induced diabetes conditions in a rat calvaria subcutaneous model.
Background
CM degradation is accelerated in uncontrolled diabetic rats. Immersion of CM in HA has been suggested to decrease their resorption rate without interfering with their tissue integration and structural degradation. However, it is unknown to what extent CM degradation may be influenced by its immersion in HA solution under a condition mimicking a medically compromised situation with an increased inflammatory level such as diabetes.
Methodology
CMs were soaked in cross‐linked HA. Protein adsorption and the HA release were quantified by ELISA. Diabetes was induced in sixteen rats, while 16 healthy rats served as control. CM was prepared and labeled prior to implantation with Biotin. Seventeen CM were immersed in HA and 17 CM in PBS. In each animal, one test or one control disk was implanted. In order to compare the collagen content, two similar non‐implanted CM were used as baseline. Fourteen days after surgery, thirty‐two animals were sacrificed. The entire calvaria including the skin above, was chemically fixed, decalcified, and embedded in paraffin. Five‐μm‐thick sections were analyzed histologically and histomorphometrically using H&E and avidin‐peroxidase staining.
Results
The in vitro results demonstrated that the CM adsorbed roughly 80% of the total HA content. After 10 days, 36.3% of the initial HA remained on the CM. The in vivo results demonstrated that diabetes significantly reduced the thickness of the CM, while HA had a significant effect on keeping the membrane thickness. HA increased the residual collagen content in the diabetic group (P < 0.0001) but no such effect was observed in the healthy group.
Conclusion
Immersion of CM in HA prior to the implantation delays membrane degradation in uncontrolled diabetic compared with normoglycemic rats.
Objective
To examine the in vitro biokinetics of hyaluronic acid (HA) from a collagen membrane (CM) and to evaluate the in vivo effect of immersion of the CM in HA solution on its degradation in streptozotocin (STZ)‐induced diabetes conditions in a rat calvaria subcutaneous model.
Background
CM degradation is accelerated in uncontrolled diabetic rats. Immersion of CM in HA has been suggested to decrease their resorption rate without interfering with their tissue integration and structural degradation. However, it is unknown to what extent CM degradation may be influenced by its immersion in HA solution under a condition mimicking a medically compromised situation with an increased inflammatory level such as diabetes.
Methodology
CMs were soaked in cross‐linked HA. Protein adsorption and the HA release were quantified by ELISA. Diabetes was induced in sixteen rats, while 16 healthy rats served as control. CM was prepared and labeled prior to implantation with Biotin. Seventeen CM were immersed in HA and 17 CM in PBS. In each animal, one test or one control disk was implanted. In order to compare the collagen content, two similar non‐implanted CM were used as baseline. Fourteen days after surgery, thirty‐two animals were sacrificed. The entire calvaria including the skin above, was chemically fixed, decalcified, and embedded in paraffin. Five‐μm‐thick sections were analyzed histologically and histomorphometrically using H&E and avidin‐peroxidase staining.
Results
The in vitro results demonstrated that the CM adsorbed roughly 80% of the total HA content. After 10 days, 36.3% of the initial HA remained on the CM. The in vivo results demonstrated that diabetes significantly reduced the thickness of the CM, while HA had a significant effect on keeping the membrane thickness. HA increased the residual collagen content in the diabetic group (P < 0.0001) but no such effect was observed in the healthy group.
Conclusion
Immersion of CM in HA prior to the implantation delays membrane degradation in uncontrolled diabetic compared with normoglycemic rats.
Purpose
To investigate clinically and radiographically at 4 months post-operatively the outcomes of mixing demineralized bovine bone material (DBBM) with cross-linked hyaluronic acid in alveolar ridge preservation.
Material and methods
Seven patients presenting bilateral hopeless teeth (14 teeth) were enrolled in the study, the test site contained demineralized bovine bone material (DBBM) mixed with cross-linked hyaluronic acid (xHyA) while the control site contained only DBBM. 4 months post-operatively prior to implant placement a Cone beam computed tomography (CBCT) scan was recorded and compared to the initial scan to assess the volumetric and linear bone resorption that occurred in both sites. Clinically, sites that needed further bone grafting at the implant placement stage were recorded. Differences in volumetric and linear bone resorption between both groups were assessed using Wilcoxon signed rank test. McNemar test was also used to evaluate difference in bone grafting need between both groups.
Results
All sites healed uneventfully, volumetric and linear resorption differences between the baseline and 4 months post-operatively were obtained for each site. The mean volumetric and linear bone resorption were respectively 36.56 ± 1.69%, 1.42 ± 0.16 mm in the controls sites and 26.96 ± 1.83%; 0.73 ± 0.052 mm in the tests sites. The values were significantly higher among controls sites (P=0.018). No significant differences were observed in the need for bone grafting between both groups.
Conclusion
Cross-linked hyaluronic acid (xHyA) appears to limit the post-extractional alveolar bone resorption when mixed with DBBM.
Background
The aim of this study was to investigate the impact of cross-linked hyaluronic acid on osteoblast-like cells seeded on top of two collagen substrates, native porcine pericardium membrane (substrate A) and ribose cross-linked collagen membranes (substrate B), in an air-lift model.
Procedure
Substrates A or B, saturated with three hyaluronic acid concentrations, served as membranes for SAOS-2 cells seeded on top. Cultivation followed for 7 and 14 days in the air-lift model. Controls used the same substrates without hyaluronic pre-treatment. Cells were harvested, and four (Runx2, BGLAP, IBSP, Cx43) different osteogenic differentiation markers were assessed by qPCR. Triplicated experiment outcomes were statistically analyzed (ANOVA, t-test; SPSS).
Outcome
Supplementary histologic analysis confirmed the cells’ vitality. After seven days, only few markers were overexpressed on both substrates. After 14 days, targeted genes were highly expressed on substrate A. The same substrate treated with 1:100 diluted xHyA disclosed statistically significant different expression level vs. substrate B (p = 0.032). Time (p = 0.0001), experimental condition as a function of time (p = 0.022), and substrate (p = 0.028) were statistically significant factors. Histological imaging demonstrated vitality and visualized nuclei.
Conclusion
We conclude that the impact of hyaluronic acid resulted in a higher expression profile of SAOS-2 cells on substrate A compared to substrate B in an air-lift culture after two weeks.