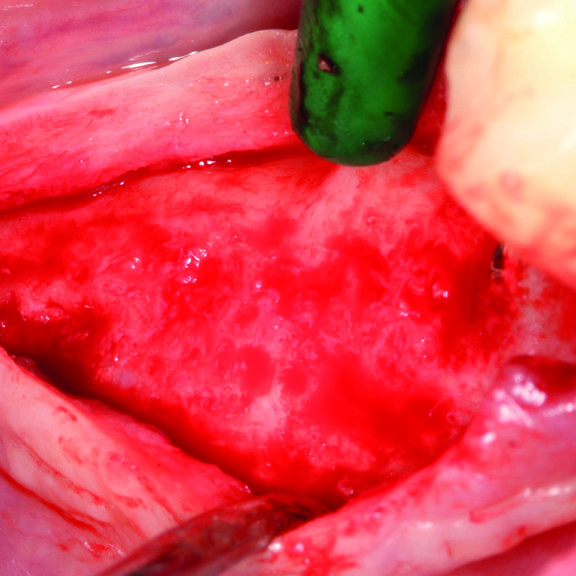
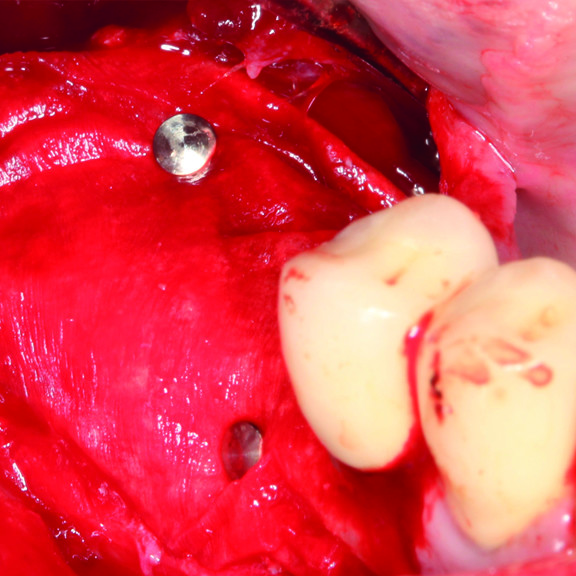
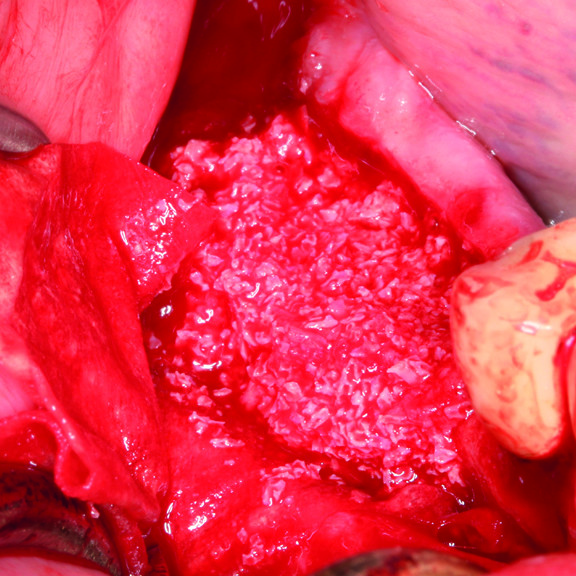
Hard Tissue Regeneration
EMPOWERING REGENERATION
At REGEDENT, we focus on leveraging hard tissue regeneration by getting artificial scaffold replaced by autogenous bone cells while providing a stable bone volume and minimizing the number of surgeries. Ultimately, patients benefit from better treatment predictability, combined with long-term outcome performance.
WHAT IS HARD TISSUE REGENERATION?
Hard tissue / bone loss is a significant hallmark in dentistry. In periodontology, for example, pathogens, genetic factors, and environmental issues, such as tobacco, can lead to hard tissue loss. It can result in tooth movement, dislocation, and eventually, tooth loss [1,2].
In implantology, the use of dental implants marks notable advantages for patients. However, it presents some risks caused by using inert materials in direct contact with bone and the absence of periodontal ligament (PDL). The PDL provides a buffer to distribute the mastication forces and, when absent, can lead to jawbone resorption [3,4].
FOUR TYPES OF HARD TISSUE REGENERATION
Various techniques are used to enhance the osteogenesis process, such as bone grafting [5], scaffolds [6], stem cells [7], and growth factors. Bone grafting helps fill the physical gaps created by the missing or damaged bone, provides structural stability, and stimulates bone tissue growth [8]. Bone grafts are divided into four groups [9, 10]. First, autogenous bone is viewed as the “gold standard” for bone replacement [11], as it is used with minimal treatment and is the most efficient and fastest-healing bone substitute material [8]. Clinical applications showed that new bone and new periodontal connective tissue attachments are obtained [12].
Second, tissue banks provide different types of allogeneic bone grafts [13]. They are known to be not as effective as autografts due to the extensive chemical treatments required to prevent potential infectivity, disrupting the hierarchical structure of bone and removing a significant amount of the growth factors necessary for efficient bone regeneration [8].
Third, xenografts show distinct advantages. They are mass-produced at affordable processing costs. However, as they originate from other species’ bone tissues, their native osteological characteristics are different from those of human bone tissues. Yet, among xenografts, some, like porcine-originated substitutes, support bone regeneration due to their physicochemical characteristics and their structure like human bone [8, 14,15]. Besides, porcine bone has a relatively low risk of zoonosis [16].
Last, synthetic graft materials, such as hydroxyapatite, are used for their osteoconduction, hardness, and acceptability by bone. Some synthetic bone grafts are made of calcium carbonate, which starts to decrease in usage because it is completely resorbable in a short time and makes the breaking of the bone easier. This last category of graft material is increasingly being researched as it presents attractive advantages that xenograft material lack.
TEN SIGNS OF SUCCESSFUL HARD TISSUE REGENERATION
- User-friendly handling of grafting material and membrane for a cost-effective, predictable treatment protocol
- Use of biocompatible material
- Rapid blood clot stabilization
- Rapid angiogenesis
- Minimize inflammatory process (swelling, discomfort)
- Uneventful wound healing
- Predictable bone density
- Stable volume for predictable implant placement
- Resorbable material to avoid further surgeries and to be replaced by autogenous cells
- Patient and clinician satisfaction
OUR HARD TISSUE REGENERATION SOLUTION
Hyadent BG, Smartbrane, and Smartgraft are designed to support hard tissue regeneration while being even more user-friendly together.
Indeed, the gel combined with the porous bone makes a sticky putty in ~3 minutes, and the membrane’s tensile strength holds the augmented site well while adapting easily to the bone surface, yet without sticking. [20] Hyaluronic acid present in the augmented site appears to accelerate bone formation through its migratory and proliferative properties. [21-24]
THE SYNERGIES BETWEEN OUR REGENERATIVE PRODUCTS
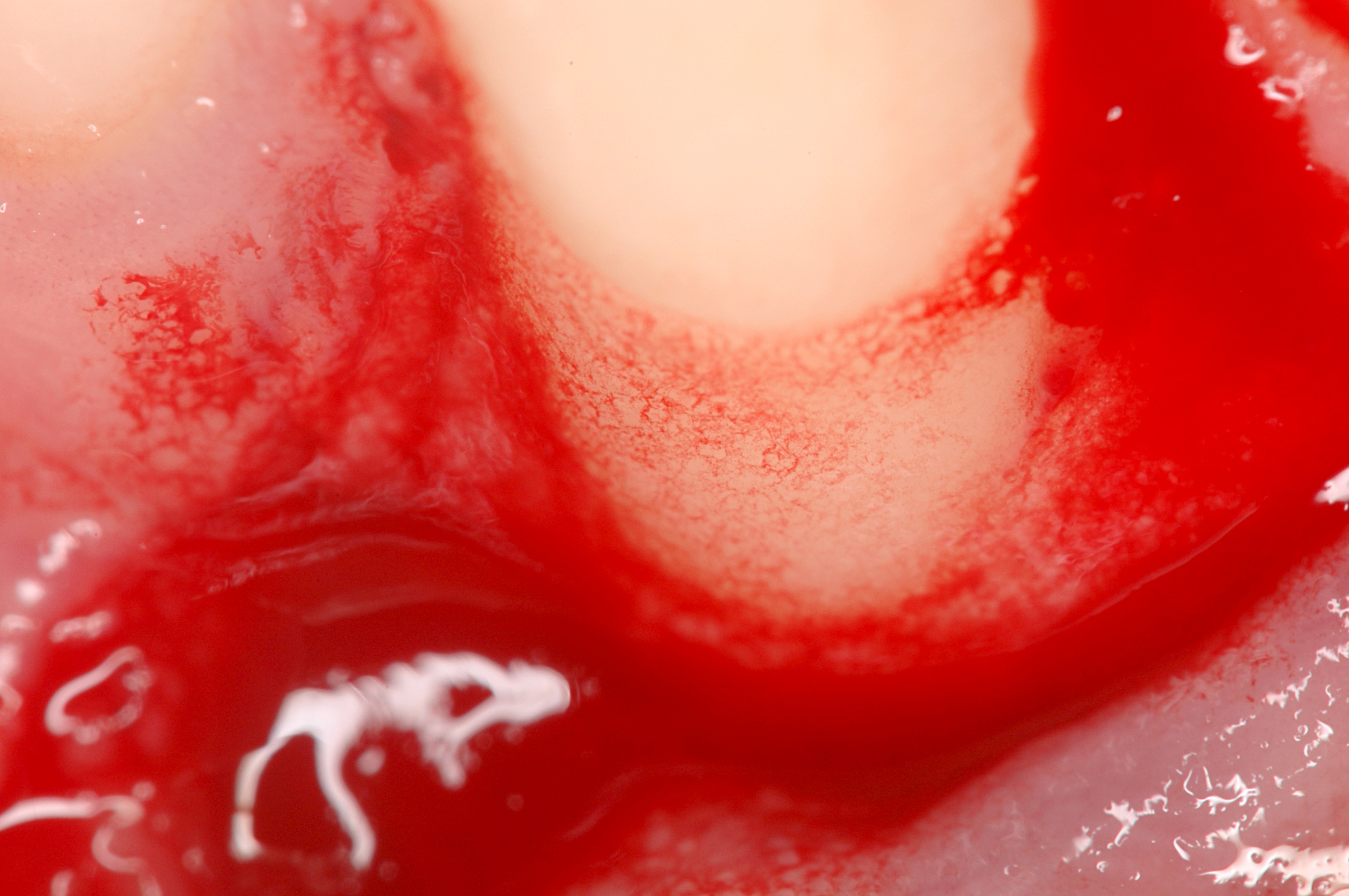
Regedent’s portfolio supports hard tissue regeneration. Hyadent BG and Smartbrane contribute to stabilizing the blood clot [25-26]. Hyaluronic acid attracts the growth factors naturally present in the blood and promotes vascularization with the support, for example, of porous porcine bone substitutes [27-29].
Bone cell adherence and proliferation are then facilitated with the porcine membrane and bone substitute’s rough surfaces [26, 28-31]. At the same time, the cross-linked high molecular weight hyaluronic acid enhances the overall cell proliferation [32-35]. Hyadent BG and Smartgraft contribute to accelerating bone healing while showing balanced bone volume remodeling [26, 36-41].
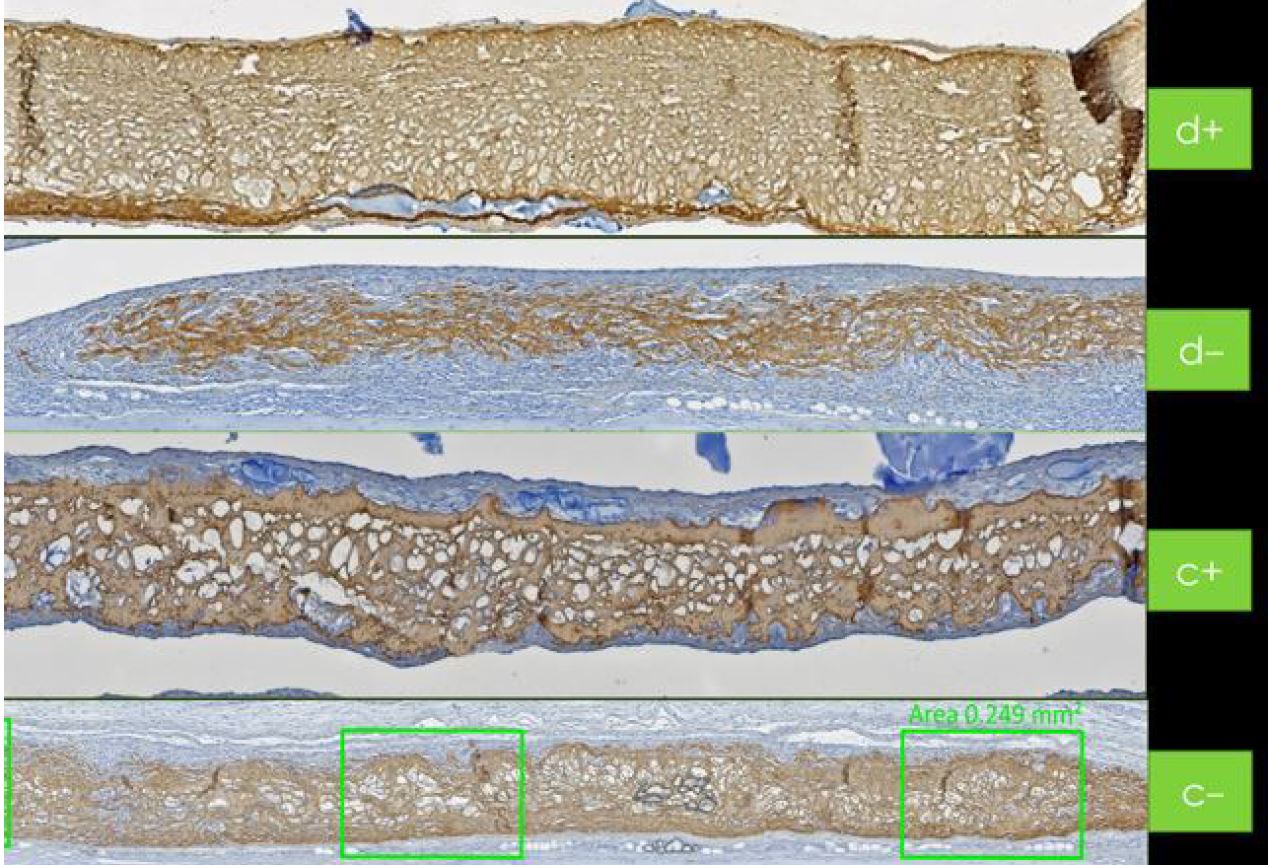
During the bone healing process, the sticky bone made from Hyadent BG and Smartgraft benefits from its natural bacteriostatic shield [42]. The strong collagen membrane holds the grafted site together during the healing process. Its maintenance function is even prolonged through the slowed-down collagenase thanks to the membrane’s earlier coating with Hyadent BG [43].
By rebuilding not only the esthetic but also the functional structure around the tooth/implant, Hyadent BG is considered to regenerate rather than repair [44-47].
The wound healing properties of the Regedent portfolio are detailed in soft tissue regeneration.
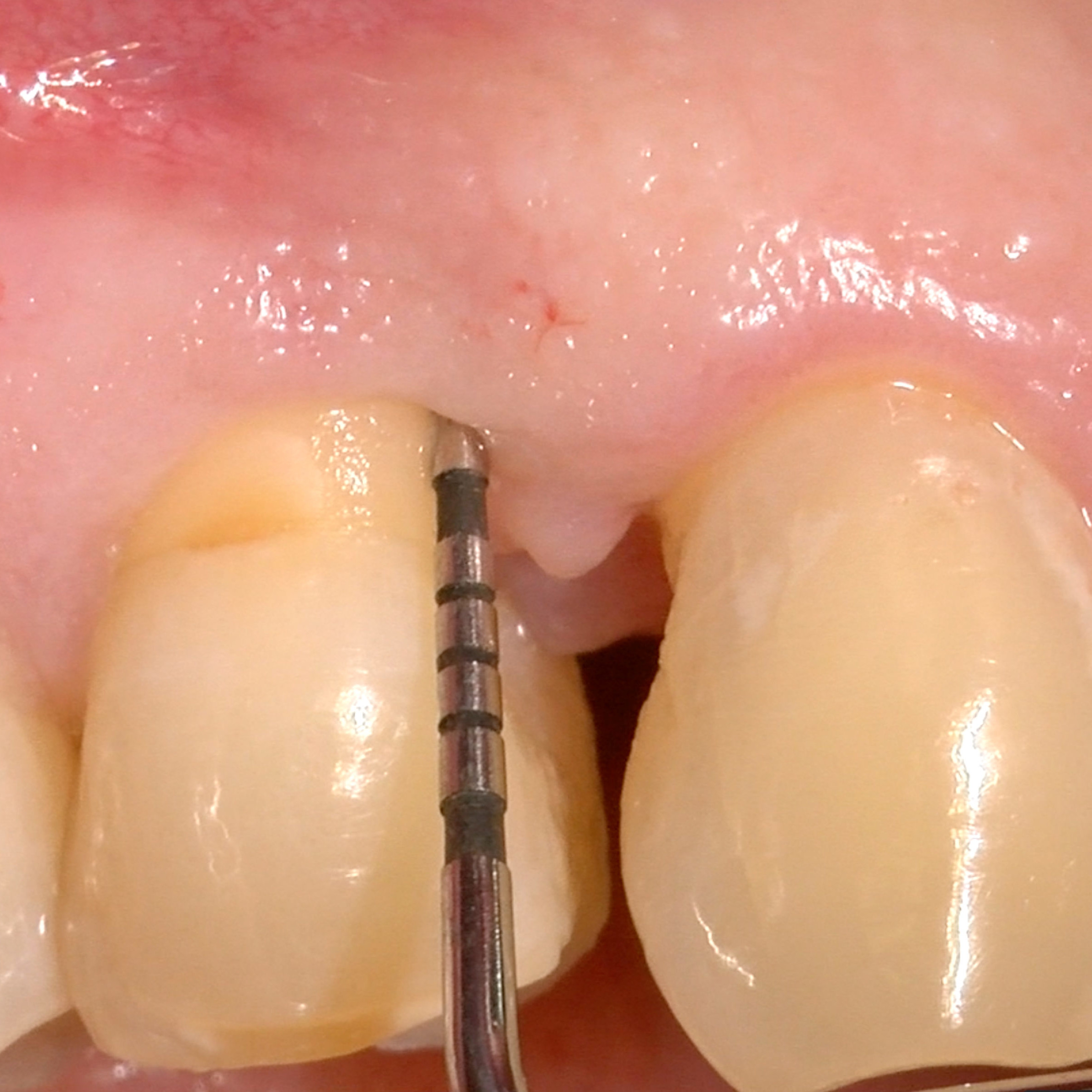
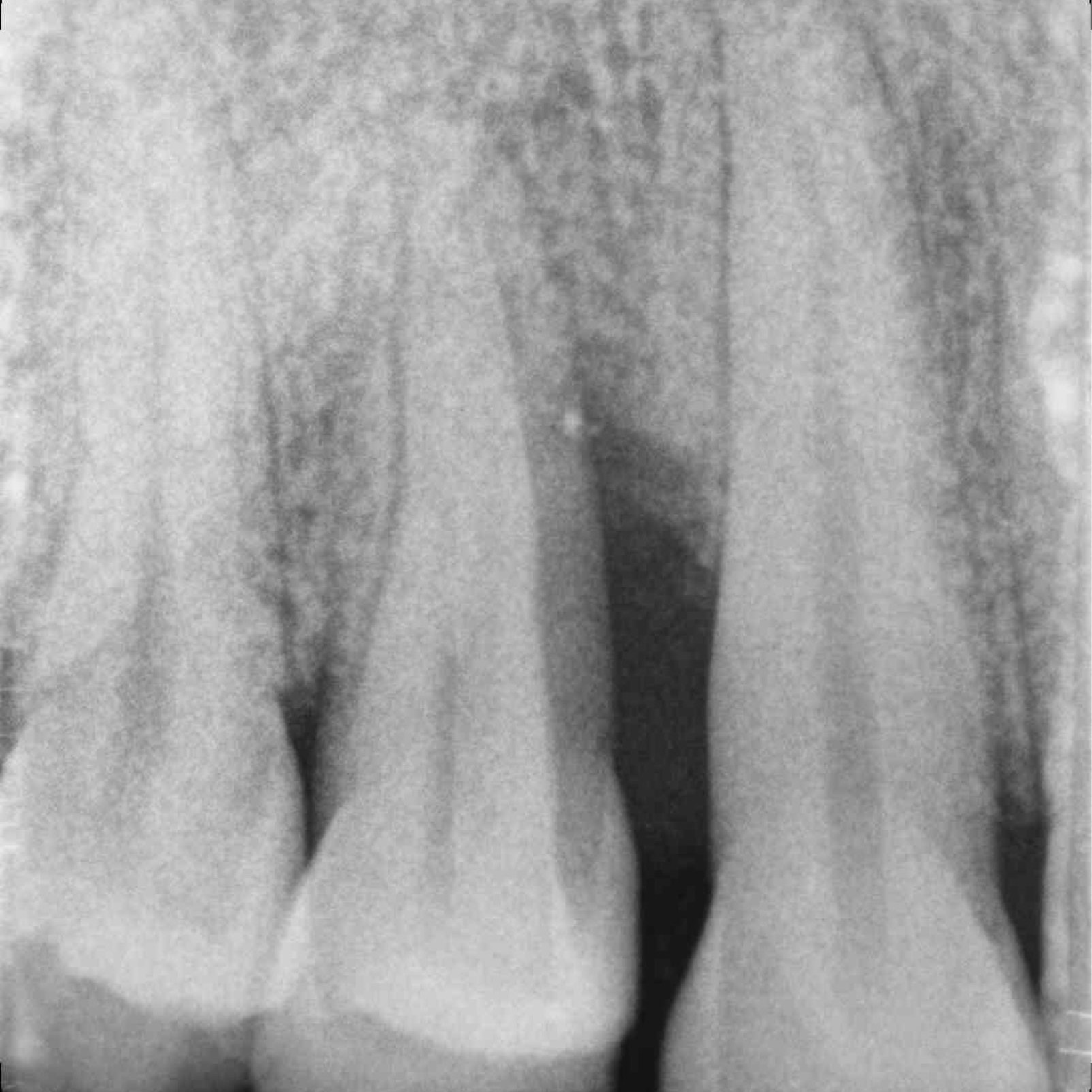
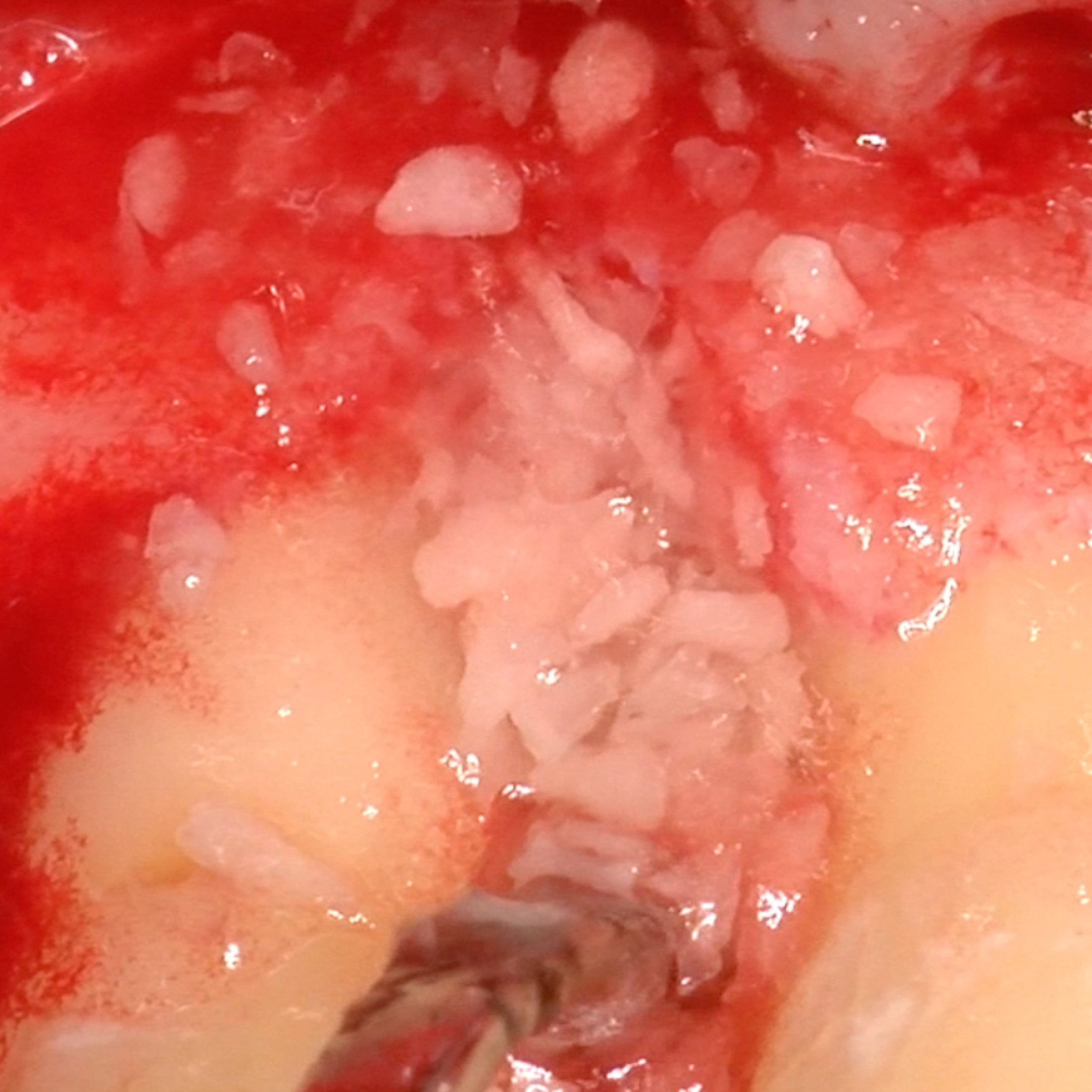
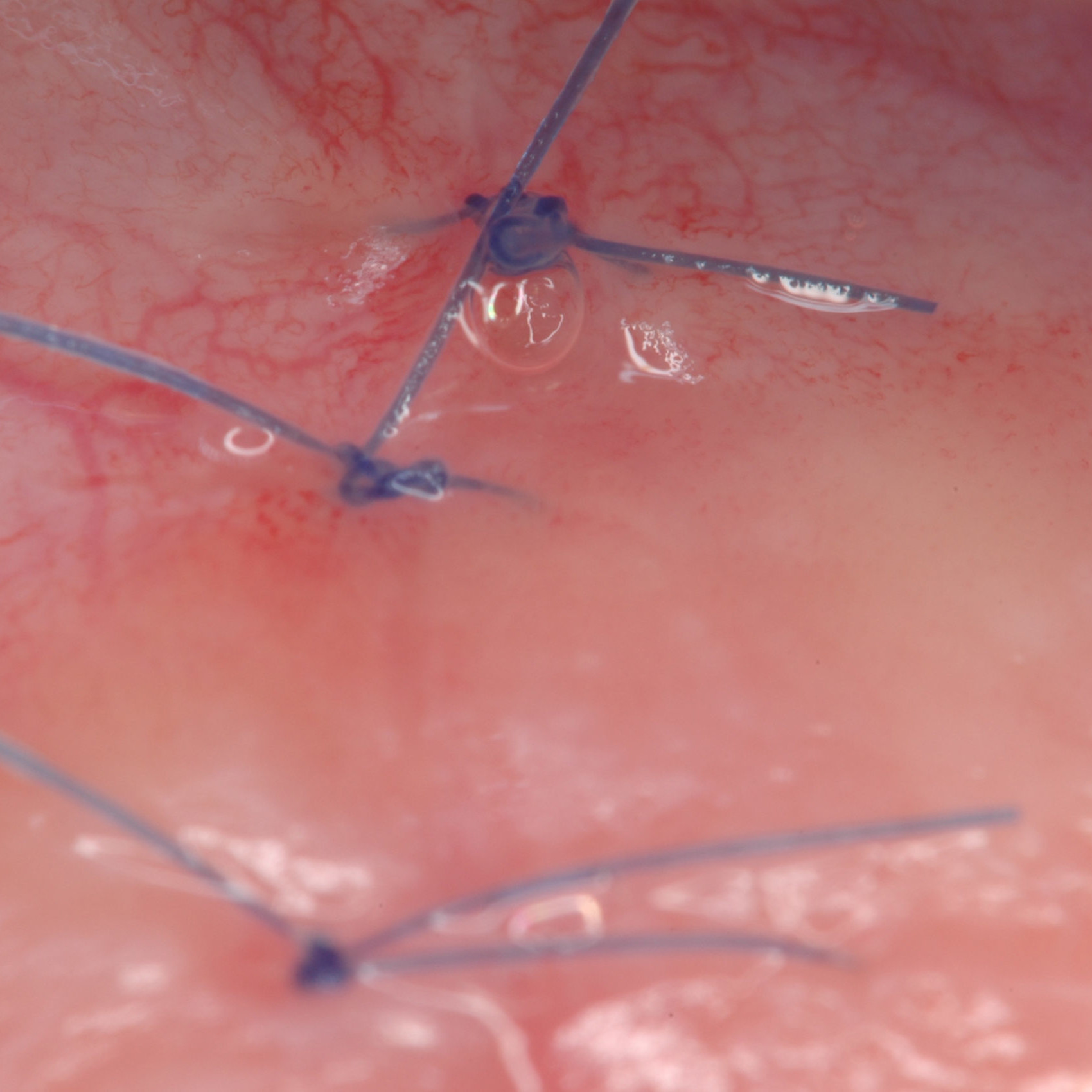
CLINICAL CASE: HARD TISSUE REGENERATION IN INFRABONY DEFECTS
Wide extended infrabony defect by Prof Andrea Pilloni with hard tissue regeneration.
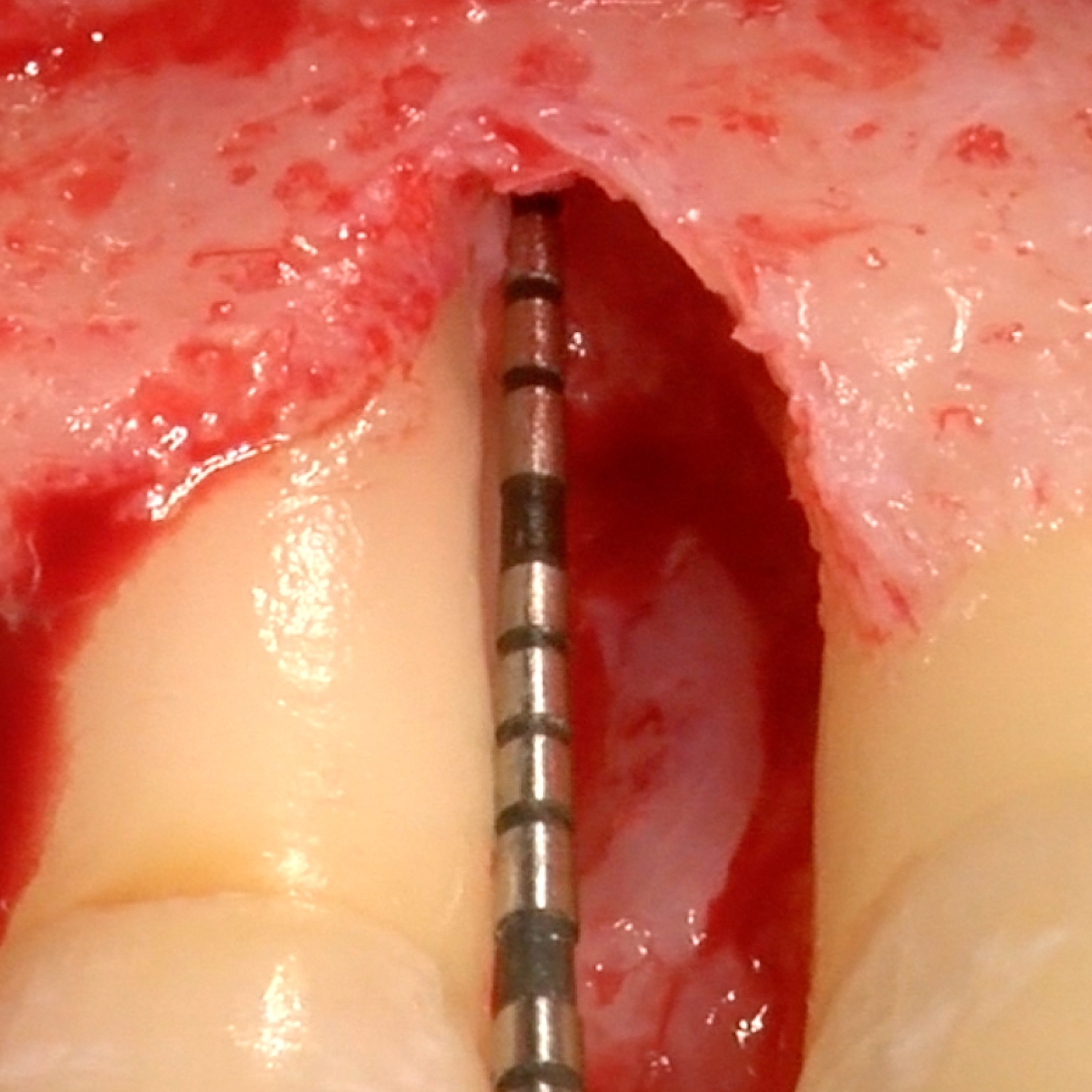
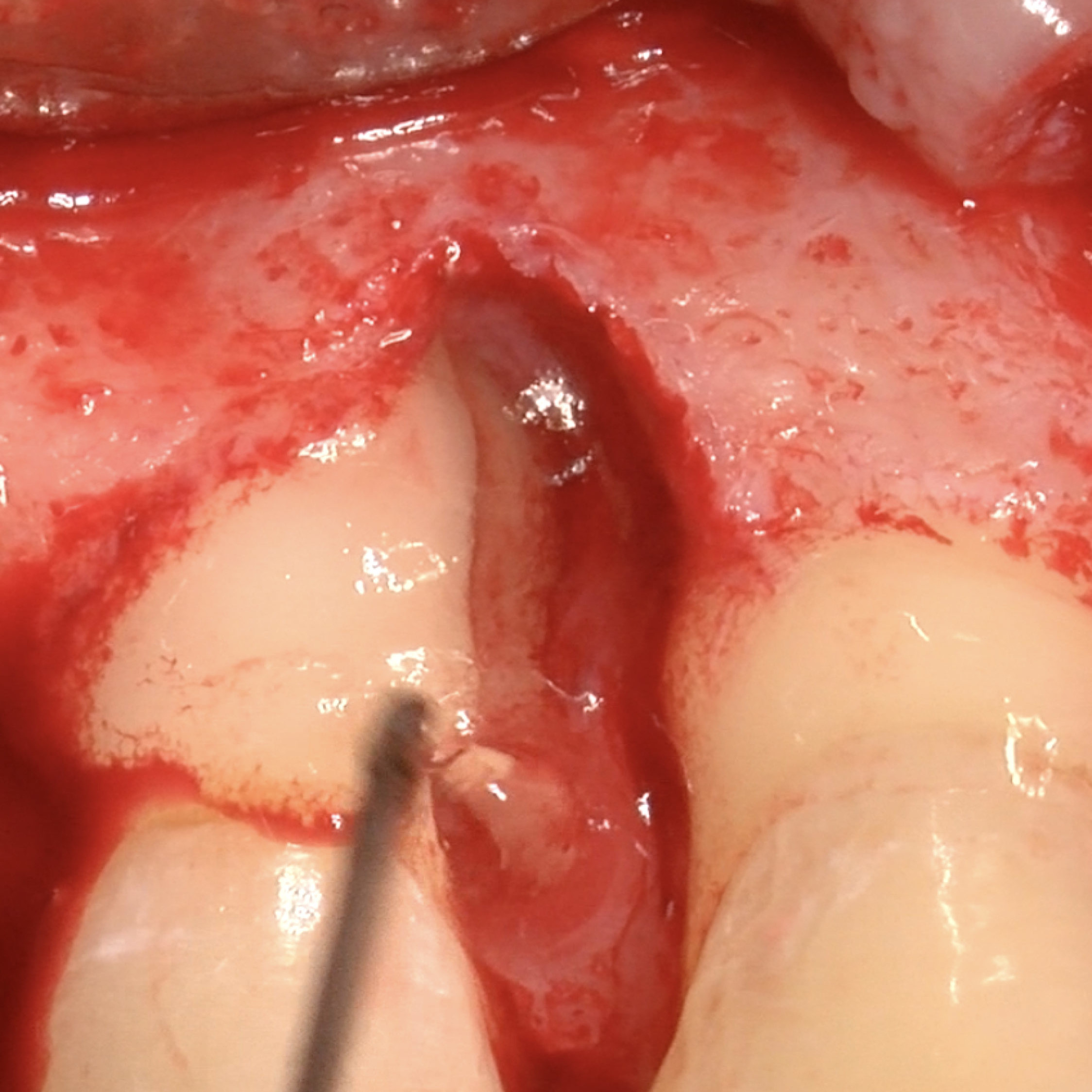
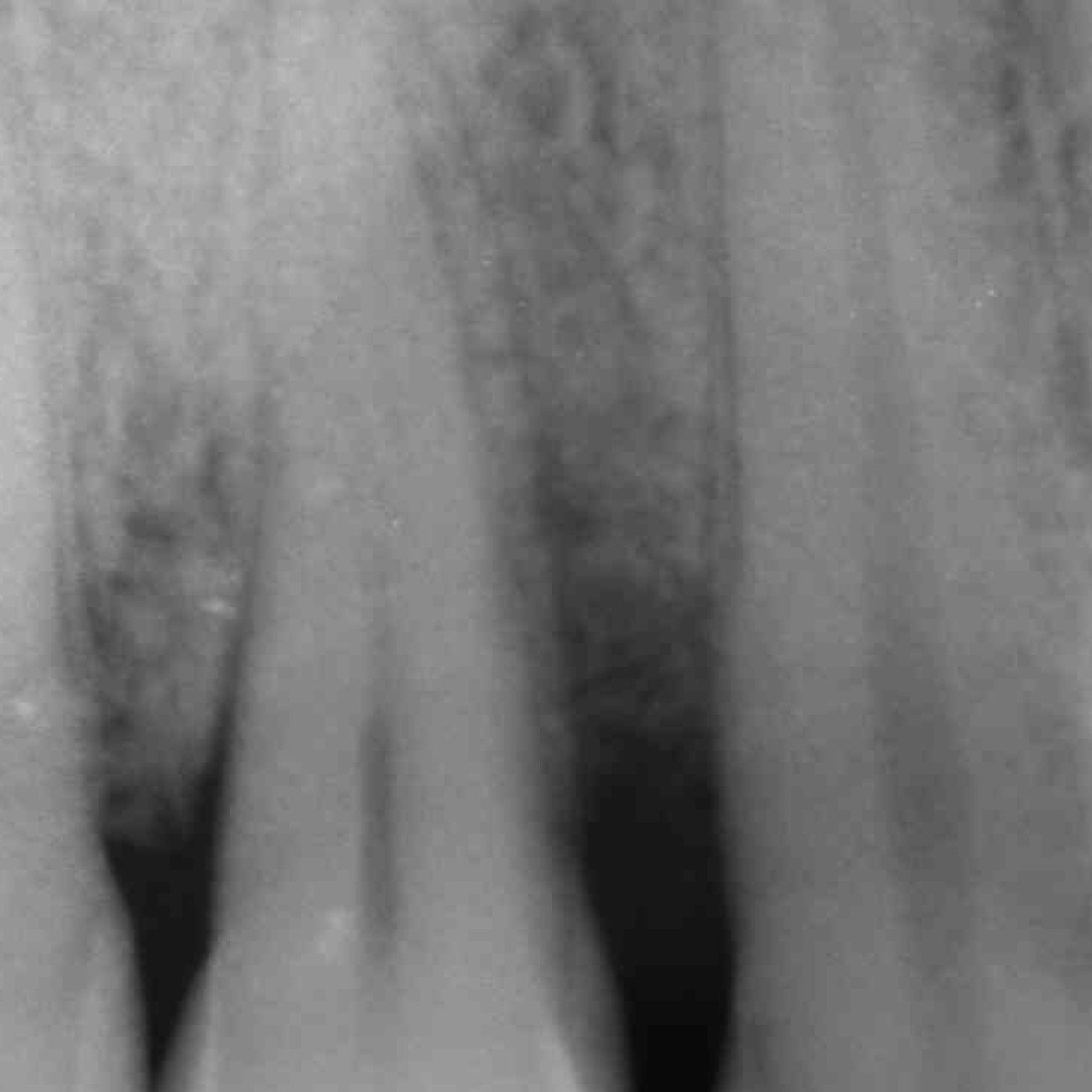
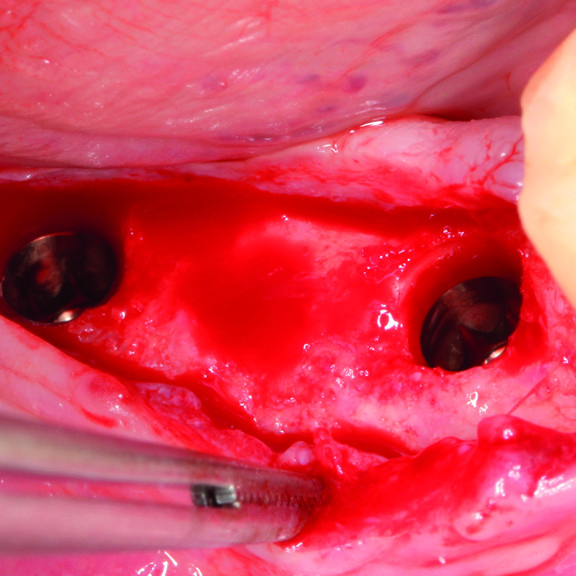
CLINICAL CASE : HARD TISSUE REGENERATION IN GBR BEFORE IMPLANT PLACEMENT
Guided Bone Augmentation of distal mandibular edentulous ridge by Prof Darko Božić with hard tissue regeneration.
SCIENTIFIC LITERATURE & CLINICAL STUDIES
- Hernández-Monjaraz B, Santiago-Osorio E, Monroy-García A, Ledesma-Martínez E, Mendoza-Núñez VM. Mesenchymal Stem Cells of Dental Origin for Inducing Tissue Regeneration in Periodontitis: A Mini-Review. Int J Mol Sci. 2018 Mar 22;19(4):944. doi: 10.3390/ijms19040944. PMID: 29565801; PMCID: PMC5979585.
- Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005 Nov 19;366(9499):1809-20. doi: 10.1016/S0140-6736(05)67728-8. PMID: 16298220.
- Hasan I, Heinemann F, Bourauel C. The relationship of bone resorption around dental implants to abutment design: a preliminary 1-year clinical study. Int J Prosthodont. 2011;24(5):457–9.
- Maiorana C, Sigurta D, Mirandola A, Garlini G, Santoro F. Bone resorption around dental implants placed in grafted sinuses: clinical and radiologic follow-up after up to 4 years. Int J Oral Maxillofac Implants. 2005;20(2):261–6.
- Anushi, M.; Suresh, K. Periodontal bone regeneration in intrabony defects using osteoconductive bone graft versus combination of osteoconductive and osteostimulative bone graft: A comparative study. Dent. Res. J. 2015, 12, 25–30.
- Zhou, M.; Geng, Y.-M.; Li, S.-Y.; Yang, X.-B.; Che, Y.-J.; Pathak, J.L.; Wu, G. Nanocrystalline hydroxyapatite-based scaffold adsorbs and gives sustained release of osteoinductive growth factor and facilitates bone regeneration in mice ectopic model. J. Nanomater. 2019, 2019, 10.
- Chen, M.; Xu, Y.; Zhang, T.; Ma, Y.; Liu, J.; Yuan, B.; Chen, X.; Zhou, P.; Zhao, X.; Pang, F. Mesenchymal stem cell sheets: A new cell-based strategy for bone repair and regeneration. Biotechnol. Lett. 2019, 41, 305–318.
- Lee JH, Yi GS, Lee JW, Kim DJ. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J Periodontal Implant Sci. 2017 Dec;47(6):388-401. https://doi.org/10.5051/jpis.2017.47.6.388
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.; Bartold, P. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221,21
- Park CH, Kim KH, Lee YM, Seol YJ. Advanced Engineering Strategies for Periodontal Complex Regeneration. Materials (Basel). 2016 Jan 18;9(1):57. doi: 10.3390/ma9010057. PMID: 28787856; PMCID: PMC5456552.
- Sakkas A, Wilde F, Heufelder M, Winter K, Schramm A. Autogenous bone grafts in oral implantology-is it still a “gold standard”? A consecutive review of 279 patients with 456 clinical procedures. Int J Implant Dent. 2017 Dec;3(1):23. doi: 10.1186/s40729-017-0084-4. Epub 2017 Jun 1. PMID: 28573552; PMCID: PMC5453915.
- Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL. Regeneration of periodontal tissue: bone replacement grafts. Dent Clin North Am. 2010 Jan;54(1):55-71. doi: 10.1016/j.cden.2009.09.003. PMID: 20103472.
- Keith, J.D., Jr.; Petrungaro, P.; Leonetti, J.A.; Elwell, C.W., Jr.; Zeren, K.J.; Caputo, C.; Nikitakis, N.G.;Schöpf, C.;Warner, M.M. Clinical and histologic evaluation of a mineralized block allograft: Results from the developmental period (2001–2004). Int. J. Periodont. Restor. Dent. 2006, 26, 320–327.
- Hölzer A, Pietschmann MF, Rösl C, Hentschel M, Betz O, Matsuura M, Jansson V, Müller PE. The interrelation of trabecular microstructural parameters of the greater tubercle measured for different species. J Orthop Res. 2012 Mar;30(3):429-34. doi: 10.1002/jor.21525. Epub 2011 Aug 10. PMID: 21834128.
- Lorenzen E, Follmann F, Jungersen G, Agerholm JS. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet Res. 2015 Sep 28;46:116. doi: 10.1186/s13567-015-0241-9. PMID: 26411309; PMCID: PMC4586017.
- Salamanca E, Lee WF, Lin CY, Huang HM, Lin CT, Feng SW, et al. A novel porcine graft for regeneration of bone defects. Materials (Basel) 2015;8:2523-36.
- Salamanca E, Lee WF, Lin CY, Huang HM, Lin CT, Feng SW, et al. A novel porcine graft for regeneration of bone defects. Materials (Basel) 2015;8:2523-36.
- Ramírez-Fernández M, Calvo-Guirado JL, Delgado-Ruiz RA, Maté-Sánchez Del Val JE, Vicente-Ortega V, Meseguer-Olmos L. Bone response to hydroxyapatites with open porosity of animal origin (porcine [OsteoBiol mp3] and bovine [Endobon]): a radiological and histomorphometric study. Clin Oral Implants Res. 2011 Jul;22(7):767-773. doi: 10.1111/j.1600-0501.2010.02058.x. Epub 2011 Jan 18. Retraction in: Clin Oral Implants Res. 2018 Jun;29(6):666. PMID: 21244497.
- Go A, Kim SE, Shim KM, Lee SM, Choi SH, Son JS, Kang SS. Osteogenic effect of low-temperature-heated porcine bone particles in a rat calvarial defect model. J Biomed Mater Res A. 2014 Oct;102(10):3609-17. doi: 10.1002/jbm.a.35022. Epub 2013 Nov 18. PMID: 24248774.].
- Internal testing results, data on file.
- Stiller M. et al. ‘Performance of β-tricalcium phosphate granules and putty, bone grafting materials after bilateral sinus floor augmentation in humans’ Biomaterials 2014;35(10):3154-3163.
- Mendes RM et al. ‘Sodium hyaluronate accelerates the healing process in tooth sockets of rat’Arch Oral Biol 2008; 53:1155–1162
- Asparuhova M, Kiryak D, Eliezer M, Mihov D, Sculean A. ‘Activity of two hyaluronan preparations on primary human oral fibroblasts’. J Periodontal Res 2018 Sep 27. Epub 2018 Sep 27
- Elkarargy A. Alveolar Sockets Preservation Using Hydroxyapatite / Beta tricalcium Phosphate with Hyaluronic Acid (Histomorphometric study). J Am Sci 2013; 9(1): 556-563]. (ISSN: 1545-1003). http://www.jofamericanscience.org. 78
- King SR, Hickerson WL, Proctor KG. Beneficial actions of exogenous hyaluronic acid on healing. Surgery 1991;109(1):76-84
- Brett D. A Review of Collagen and Collagen-based Wound Dressings. Wounds 2008;20(12).
- King, S.R., Hickerson, W.L. and Proctor, K.G. (1991) Beneficial Actions of Exogenous Hyaluronic Acid on Wound Healing. Surgery, 109, 76-86.
- Deligianni DD, Katsala ND, Koutsoukos PG, Missirlis YF, Effect of Surface Roughness of Hydroxyapatite on Human Bone Marrow Cell Adhesion, Proliferation, Differentiation and Detachment Strength. Elsevier Biomaterials 22 (2001) 87–96 2.
- Shu-Thung L et al. (2014) Isolation and Characterization of a Porous Carbonate Apatite From Porcine Cancellous Bone. Science, Technology, Innovation, Aug: 1-13 Brett D. A Review of Collagen and Collagen-based Wound Dressings. Wounds 2008;20(12)
- Nichols A, Burns DC, Christopher R. Studies on the Sterilization of Human Bone and Tendon Muscoskeletal Allograft Tissue Using Supercritical Carbon Dioxide. Journal of Orthopaedics 2009.
- Sawada K, Terada D, Yamaoka T, Kitamura S, Fujisato T. Cell removal with supercritical carbon dioxide for acellular artificial tissue. J Chemical Technol Biotechnol 2008;83(6):943–949.
- Fawzy ES. et al. Local application of hyaluronan gel in conjunction with periodontal surgery: a randomized controlled trial.Clin Oral Invest 2012;16:1229-1236
- Briguglio, F. et al. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid:A randomized clinical trial. Quintessence Int2013;44:231-240
- Kessiena L. Aya et al. ‘Hyaluronan in wound healing: Rediscovering a major player.’ Wound Rep Reg 2014;22:579-593.Dental Journal. (2017) Vol.42:104-11.
- Sasaki T, Watanabe C, Stimulation of Osteoinduction in Bone Wound Healing by High-Molecular Hyaluronic Acid. Bone. Vol. 16. No.1 January 1995:9-15
- Ghada Bassiouny A. ‘Bioinspired Approach for Dental Implant Fuctionalization: An Experimental Study Evaluating the Effect of Hyaluronate as Bioactive Implant Coating.’ J Am Sci 2013;9(11):187-192]. (ISSN: 1545-1003). http://www.jofamericanscience.org. 25
- Shamma MM, Ayad SS, El-dibany RM, Nagui DA ‘Evaluation of the effect of hyaluronic acid mixed with biphasic calcium phosphate on bone healing around dental implants’ Alexandria Dental Journal. (2017) Vol.42 Pages:104-11
- Kim JJ, Song HY, Ben Amara H, Kyung-Rim K, Koo KT. Hyaluronic Acid Improves Bone Formation in Extraction Sockets With Chronic Pathology: A Pilot Study in Dogs. J Periodontol. 2016;87(7):790-795. doi:10.1902/jop.2016.150707
- Bracey DN, Seyler TM, Jinnah AH, Lively MO, Willey JS, Smith TL, et al. A decellularized porcine xenograft-derived bone scaffold for clinical use as a bone graft substitute: a critical evaluation of processing and structure. J Funct Biomater. 2018;9(3):45.https://doi.org/10.3390/jfb9030045
- Renzo et al.: Tissue Dimensional Changes Following Alveolar Ridge Preservation with Different Xenografts Associated with a Collagen Membrane. Results at the 4-Month Re-Entry Surgery. Int Arch Oral Maxillofac Surg, 2017, 1:003 12.
- Guarnieri R, Di Nardo D, Di Giorgio G, Miccoli G, Testarelli L. Effectiveness of Xenograft and Porcine-Derived Resorbable Membrane in Augmentation of Posterior Extraction Sockets with a Severe Wall Defect. A Radiographic/Tomographic Evaluation. J Oral Maxillofac Res. 2019 Mar 31;10(1):e3. doi: 10.5037/jomr.2019.10103. PMID: 31086644; PMCID: PMC6498814
- Pirnazar P. et al. ’Bacteriostatic effects of hyaluronic acid.’ Journal of Periodontology 1999;70:370-374
- Eliezer M, Sculean A, Miron RJ, et al. ‘Hyaluronic acid slows down collagen membrane degradation in uncontrolled diabetic rats.’ J Periodontal Res. 2019;00:1–9. https ://doi.org/10.1111/jre.12665
- Longaker T et al. ‘Studies in Fetal Wound Healing: V. A prolonged presence of hyaluronic acid characterizes fetal wound healing.’ Ann. Surg. 1991; April:292–296.
- Mast BA et al. ‘Hyaluronic Acid Modulates Proliferation, Collagen and Protein Synthesis of Cultured Fetal Fibroblast.’ Matrix,1993;13:441–446.
- Asparuhova MB et al.. ‘Activity of two hyaluronan preparations on primary human oral fi broblasts.’ J Periodont Res.2018;00:1–13.
- Salbach J et al. ‘Regenerative potential of glycosaminoglycans for skin and bone.’ J Mol Med 2012;90:625–635.