Table of contents
- What is cross-linked hyaluronic acid (xHyA)?
- What are the differences between hyaluronic acids used for dental treatments?
- Why is hyaluronic acid (HyA) relevant in oral wound healing?
- How did hyaluronic acid pioneer in dentistry?
- Why is hyaluronic acid used in periodontology?
- Why is hyaluronic acid used in GBR and dental surgery?
- What are the advantages of combining native and cross-linked hyaluronic acid in dental surgery?
- Conclusion: The reasons for using cross-linked hyaluronic acid in dental treatments
- References
What is cross-linked hyaluronic acid (xHyA)?
Hyaluronic acid, also known as hyaluronan and abbreviated as HA or HyA, that is used for dental treatments as well as in other medical and cosmetic applications, is a naturally occurring non-sulphated glycosaminoglycan. It was first isolated from the vitreous humor of the eye by Meyer and Palmer in 1934.1 Cross-linked hyaluronic acid (xHyA) is generated by a process which forms new bonds between linear / native HA, thus joining molecules together (cross-linking); see below for more details on its properties and applications.
Physiologic function of hyaluronic acid
Hyaluronic acid possesses important hygroscopic, rheological, and viscoelastic properties and thus features numerous physiological and structural functions. Aside from its well-known role as a lubricant in joints, it plays a significant role in fundamental regenerative processes such as wound healing and embryogenesis. HA is particularly prominent during instances of rapid tissue turnover and repair.
HA is involved in maintaining the resilience and tissue hydration of the extracellular matrix (ECM)and is widely distributed throughout the body, with the skin containing approximately half of it. In periodontal tissues, HA is found in higher quantities in the gingiva and the alveolar ligament2,3.
Its biological effects are closely related to its form (full molecular chain or fragments) and molecular weight. In its complete form, HA has both hydrophilic and hydrophobic properties: it can capture or release molecules from the environment (water, ions, growth factors). HA with a molecular weight between 0.4 and 4 kDa acts as an inducer of inflammation, while HA with a molecular weight between 20 and 200 kDa participates in significant biological mechanisms such as embryogenesis and wound healing. HA with a molecular weight above 500 kDa plays an anti-angiogenic and filling role.4 In the absence of any wound or healing phenotype, HA is present in the form of long chains of high molecular weight, contributing to the harmonious functioning of the extracellular matrix. It plays a structural role, regulates tissue hydration and plasticity, controls cell-cell interactions, and has anti-inflammatory properties. As an essential component of the extracellular matrix, HA also plays a pivotal role in the wound healing process.5
What are the differences between hyaluronic acids used for dental treatments?
Hyaluronic acid is not a single molecule, but rather a family of molecules with different features. The differences are numerous and can lead to various effects. Here are some key characteristics:
Low molecular weight vs. high molecular weight
The physiological functions of HA depend on its molecular weight (MW), which is determined by the length of the HA chain. High molecular weight (long chain) hyaluronic acid is generally involved in the modulation of the immune response pathway through its immunosuppressive, antiangiogenic, and anti-inflammatory properties.
Low molecular weight (short chain) HA is relevant to wound and tissue healing as it exhibits angiogenic, immunostimulatory and inflammatory properties.6,7
Purity
The purity may vary significantly depending on its intended use. There are notable differences in the quality of HA used in cosmetic, or surgical/pharmaceutical applications. Typically, those manufactured for surgical/pharmaceutical purposes adhere to the highest standards and offer the best
Native/linear versus cross-linked hyaluronic acid (xHyA)
The choice between native and cross-linked hyaluronic acid depends on the specific indication and desired function. Native HA retains its natural form and exhibits the highest regenerative potential. It is naturally degraded in the body within a few hours to a few days.
Cross-linked hyaluronic acid (xHyA) involves additional chemical modifications to extend its degradation behaviour, which can range from several weeks to months. However, as hyaluronic acid becomes cross-linked, its physiological properties are reduced, and it becomes more inert. It’s important to consider the specific requirements and intended application when formulating the appropriate type of HA for medical devices.
Composition of hyaluronic acid-based gels
In addition to the previously mentioned factors, HA-based gels used for specific indications can vary in terms of several factors:
- Concentration: The concentration of HA in the gel can impact its viscosity and potentially its efficacy. Higher concentrations often result in higher viscosity and may enhance the performance of the device.
- Composition: HA formulations can consist of a single type of HA that specifically targets a particular tissue or healing pattern. Alternatively, there may be formulations that contain multiple types of HA, each targeting different categories of healing patterns or tissues. For example, there could be variations in HA formulations that are specific to gingival cells versus bone cells.
- Additional substances: HA formulations may include additional substances such as disinfectants, peptides, polynucleotides, mannitol or viscosity modifiers. These substances can potentially interact with the beneficial properties of HA, such as impacting its resorption pattern, regenerative effect… It is essential to carefully consider:
- the biological requirement of the indication (e.g. bone augmentation requires a lasting effect vs stimulation of gingival wound healing that will be over few days)
- the formulation must match the specificity of the application (e.g. gel for direct application into the surgical site, combination of the HA with other biomaterial or a non-surgical application for periodontal pockets)
- potential interactions to ensure the desired outcomes (e.g. high molecular weight cross-linked hyaluronic acid slows the resorption of collagen (i.e. collagen membrane)
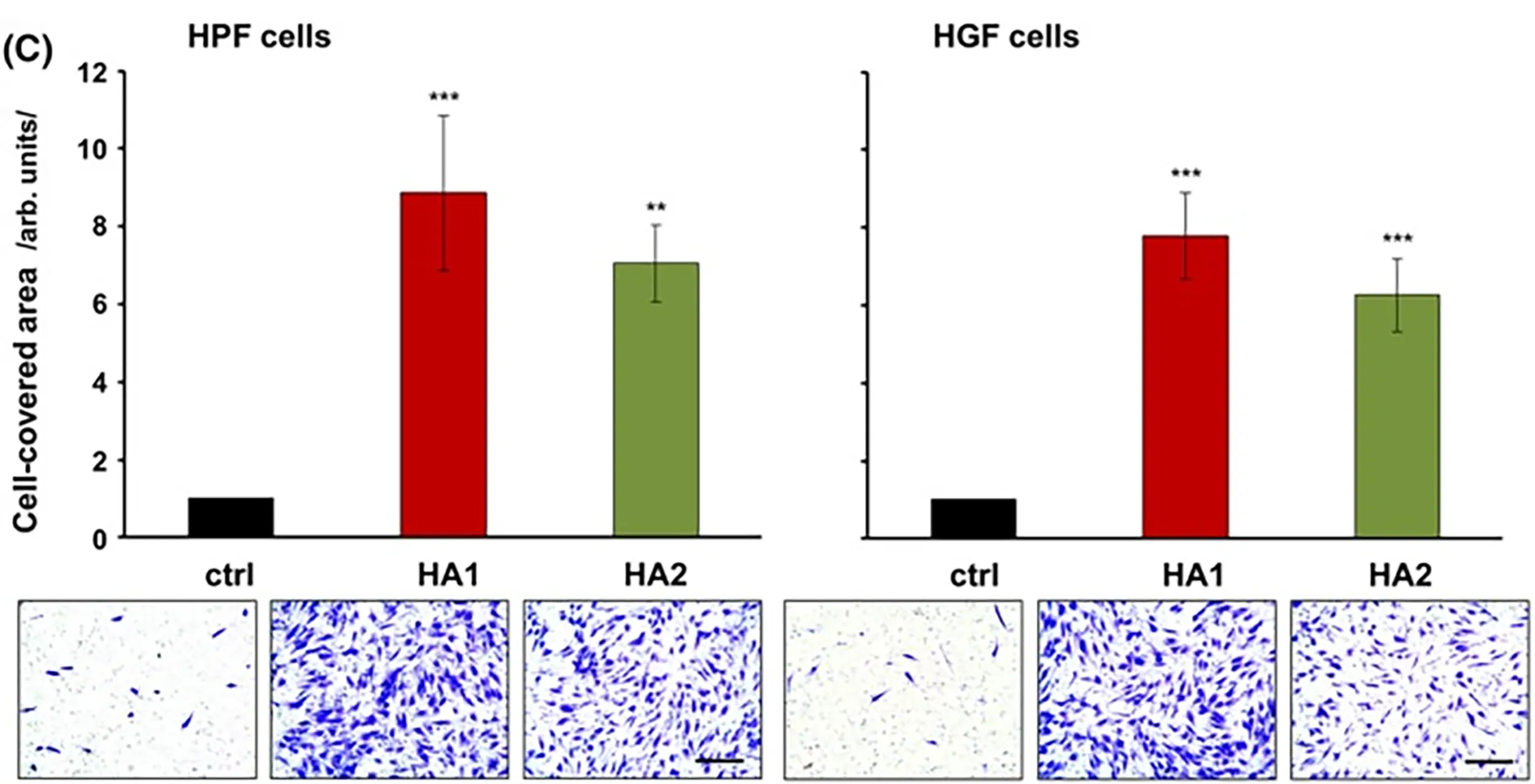
The figures below demonstrates the impact of different degrees of cross-linking on the proliferation of palatal and gingival cells.8
Why is hyaluronic acid (HA) relevant in oral wound healing?
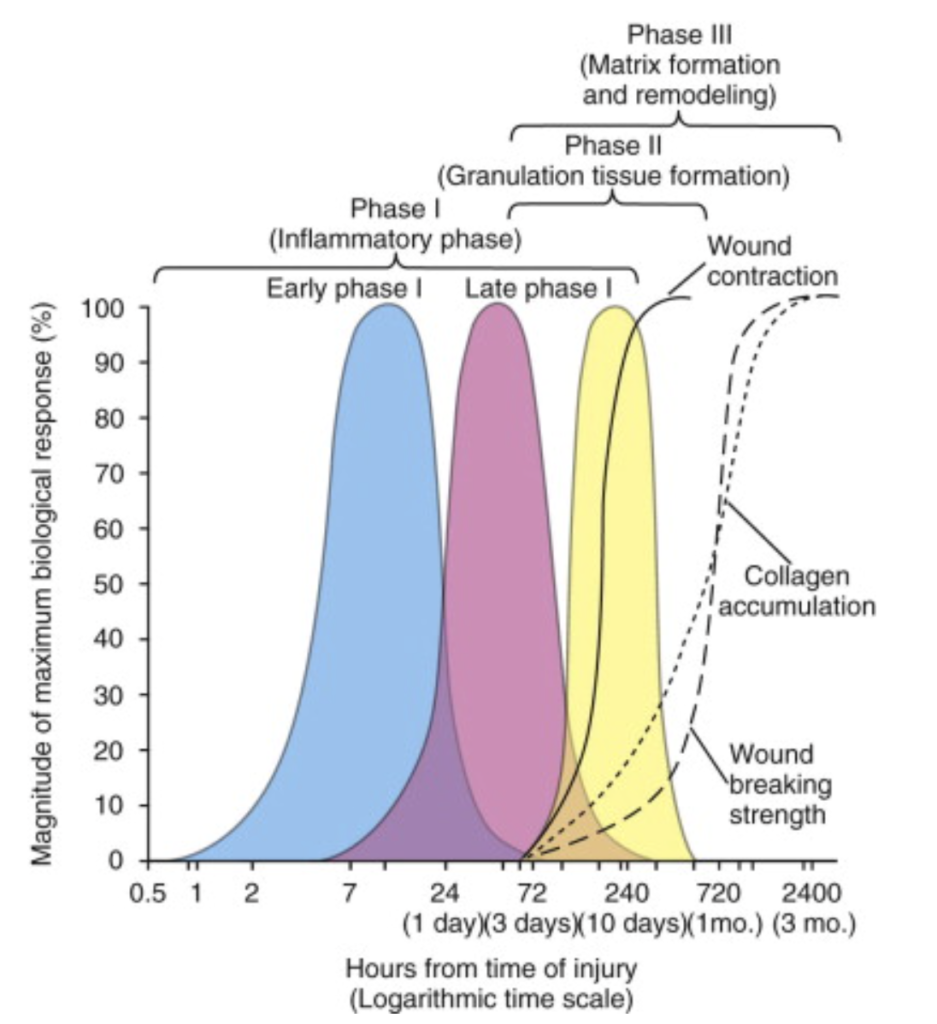
When an oral wound occurs, HA is one of the first substances to be present at the site. It contributes to the initial stages of wound healing. HA is cut into small fragments by special enzymes (hyaluronidases) and exhibits differential effects based on its molecular weight (MW).9 In this form of short chains (oligomers) it binds to different receptors and becomes a key regulatory agent involved in clot formation, the inflammatory process, angiogenesis, repair, and tissue maturation.10
How did hyaluronic acid pioneer in dentistry?
By the usage of hyaluronic acid (HA), significant advancements could be achieved in the field of dentistry, pioneering new applications and techniques. Due to its versatile properties, hyaluronic acid has found extensive use in biomedical applications, particularly in dermatology and aesthetic medicine, such as dressings, patches, and injections.
Hyaluronic acid for dental papilla?
In dentistry, the initial attempts to utilize HA focused on increasing the size of interdental papilla, commonly known as the “black triangle” between teeth. The success of HA injections in augmenting interdental papillary volume varies on several factors. These include the severity of papillary loss, the papilla morphology, injection technique, concentration and amount of HA used, as well as patient-related factors like smoking and oral hygiene.
Several studies have investigated the effectiveness of HA injections in increasing interdental papillary volume, and the outcomes have shown significant variation. Recent studies,11 along with a systematic review12 and meta-analysis of 12 studies reported an overall mean increase in papillary height of 1.25 mm. The success rate ranged from 31.5% to 100%, and the duration of the effect varied from 6 to 18 months.
While HA injections for interdental papilla augmentation can be successful, there are potential side effects to consider. Mild to moderate pain, swelling, bruising, and bleeding at the injection site have been documented. In rare cases, more severe adverse events like infection, allergic reactions, and tissue necrosis have been reported.
Although several companies have explored this area, there is currently no conclusive and predictable therapy based on HA injection in the interdental papilla. Further research and development are necessary to optimize techniques and establish more definitive treatment approaches.
Hyaluronic acid for Periodontology and Guided Bone Regeneration (GBR)
The use of hyaluronic acid in dental applications has gained significant momentum, thanks to the extensive research conducted by international teams of researchers. These studies have explored various areas within dentistry, leading to valuable insights and advancements. Here are some notable research teams and their contributions:13
- Prof Anton Sculean (Bern, Switzerland) and Prof Andrea Pilloni (Rome, Italy): Their work has focused on periodontal surgical regeneration and plastic-aesthetic surgery. They have conducted pre-clinical and clinical studies in this field, contributing to the understanding and application of HA in these areas.
- Prof Stefan Fickl (Würzburg, Germany) and Prof Darko Bozic (Zagreb, Croatia): These research teams have been active in the area of guided bone regeneration (GBR) research. Their studies have investigated the use of HA and its potential benefits in promoting bone regeneration during dental procedures.
- Prof Anton Friedmann (Witten-Herdecke, Germany) and Prof Vincenzo Iorio-Siciliano (Naples, Italy): These teams have focused on non-surgical periodontal infection treatment as well as surgical and non-surgical peri-implant infection treatment. Their research has explored the application of HA in managing and treating various types of periodontal and peri-implant infections.
Why is hyaluronic acid used in periodontology?
The use of HA in periodontology holds promise for promoting periodontal regeneration, reducing pocket depth, improving clinical attachment, and providing a convenient alternative or adjunct to traditional treatments. Further research and clinical studies continue to explore the full potential of HA in periodontal therapy.
Regenerative potential: HA, particularly when used in combination with bone substitutes, has shown promising results in the regeneration of sub-osseous periodontal lesions. Pre-clinical studies have demonstrated that a gel containing cross-linked and native HA significantly contributes to the regeneration of the periodontium, including cementum, periodontal ligament, and alveolar bone. This approach has shown superior tissue generation compared to traditional methods that involve using collagen membranes to isolate the periodontium from epithelial cells.14,15,16
Comparable effectiveness: HA has been shown to have clinical effects comparable to enamel matrix derivatives (EMD, Emdogain), which are widely recognized as effective bio-inducing agents in periodontal regeneration.17
PD, BOP, CAL improvement: Studies have indicated that HA can reduce periodontal pocket depth and improve clinical attachment, making it a favourable alternative or adjunct to traditional treatments.18,19,
Ease of application: HA is easy to apply in surgical settings without the need for extensive site preparation and even in the presence of blood or saliva. This makes it a convenient option compared to other biologics, such as enamel matrix derivatives, used in periodontal procedures.
Extension to mucogingival surgery: HA has been increasingly used in mucogingival surgery alone and as adjunct to connective tissue grafting, free gingival grafting. Similar to the role of enamel matrix derivatives in the past, HA can be utilized to stabilize the clot and promote tissue integration between the root surface, flap, and connective tissue graft.20
Why is hyaluronic acid used in GBR and dental surgery?
Hyaluronic acid has found applications in guided bone regeneration (GBR) and dental surgery due to its beneficial effects on graft stabilization “sticky bone”, better graft volume stability during the remodeling, new bone cells and the improved healing processes. Here’s why HA is used in these contexts:
- Impact on bone cells: Initial evidence in periodontology, particularly from the research team of Prof Anton Sculean, has shown that cross-linked hyaluronic acid (xHyA) has a positive impact on cells such as periodontal ligaments, cementum, and alveolar bone. The administration of HA has been observed to stimulate the proliferation of mesenchymal cells, attract growth factors, and promote angiogenesis.22,23,24 This has led to further exploration of its effects on bone cells and its combination with bone graft substitutes.
- Combination with bone graft substitutes: In clinical settings, the combination of HA with bone graft substitutes, such as deproteinized bovine bone mineral (DBBM), has been evaluated.21 To overcome the rapid disintegration of fast-resorbing natural HA, cross-linked hyaluronic acid (xHyA) has been introduced with a slower degradation profile, allowing for a longer presence and impact, especially in biological processes like bone healing.25
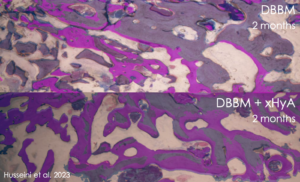
- Improved bone regeneration: Studies, including a split-mouth case series by Dr. Husseini, have shown that combining cross-linked hyaluronic acid (xHya) with DBBM as a slow-resorbing bone graft material resulted in significantly lower volumetric resorption of the buccal bone profile compared to using DBBM alone. This indicates that the addition of xHyA reduced the need for additional bone grafting. Histological evaluations have also demonstrated higher levels of new bone formation and better bone quality in the cross-linked hyaluronic acid group compared to the group treated with DBBM alone. In vitro studies have provided a biological rationale for these findings, highlighting the stimulating role of hyaluronic acid in osteoblastic activity.26,27,28,29
The use of HA, particularly cross-linked hyaluronic acid, in GBR and dental surgery aims to enhance bone regeneration, improve bone quality, and optimize the healing process. Ongoing research and clinical experiments continue to explore the full potential and benefits of HA in these applications.
Cross-linked hyaluronic acid for “sticky bone” in GBR
 Cross-linked hyaluronic acid (xHyA) plays a significant role in achieving “sticky bone” and supporting the primary stability of augmented sites when combined with bone graft materials. The stability of the wound is crucial for volume-stable bone regeneration, and it relies on the ability of the bone graft particles to remain in place during the healing process. Studies have shown that early wound closure can cause displacement of the bone substitute, leading to partial collapse of the augmented volume in the coronal portion of the recipient site.30 If the bone graft particles are not stable and migrate away from the defect site, the augmented volume is reduced, compromising the effectiveness of the guided bone regeneration (GBR) procedure.
Cross-linked hyaluronic acid (xHyA) plays a significant role in achieving “sticky bone” and supporting the primary stability of augmented sites when combined with bone graft materials. The stability of the wound is crucial for volume-stable bone regeneration, and it relies on the ability of the bone graft particles to remain in place during the healing process. Studies have shown that early wound closure can cause displacement of the bone substitute, leading to partial collapse of the augmented volume in the coronal portion of the recipient site.30 If the bone graft particles are not stable and migrate away from the defect site, the augmented volume is reduced, compromising the effectiveness of the guided bone regeneration (GBR) procedure.
To ensure graft stability, it is essential to use bone graft materials with appropriate particle size and shape, and to properly placed and stabilized the graft with a barrier membrane during the procedure. The adjunction of the cross-linked hyaluronic acid gel, which has a prolonged resorption pattern of several weeks, contributes in different ways. The gel acts as a liquid spacer between the bone graft particles, filling the void and reducing the collapse of the augmented volume compared to using loose bone graft particles hydrated only with physiological solution. Being a hydrophilic material, cross-linked hyaluronic acid attracts blood, stabilizes the clot and helps hold stabilize the particles in place. It also interacts/attracts the relevant bone cells, promoting faster and better bony integration of the bone graft particles into the surrounding bone tissue.
What are the advantages of combining native and cross-linked hyaluronic acid in dental surgery?
The combination of native/linear hyaluronic acid with cross-linked hyaluronic acid gel into a high concentration gel, offers numerous advantages appreciated in dental surgery. Here are the key benefits appreciated in this field:
- Prolonged presence: The cross-linking of hyaluronic acid extends its presence in the oral cavity for several weeks. This duration is crucial for impacting different oral cell types involved in the healing process.
- Hydrophilic property: The hydrophilic nature of cross-linked hyaluronic acid (xHya) attracts blood and helps stabilize the blood clot when applied topically to surgical sites. This promotes a favourable environment for healing.
- Enhanced cell attachment: The cross-linked hyaluronic acid creates an ideal surface topography that facilitates advanced cell attachment, promoting tissue integration and regeneration.31
- Reduced inflammation: xHyA has been shown to down-regulate the inflammatory process, resulting in reduced swelling, less pain, and faster wound epithelialization. These effects contribute to improved patient comfort and recovery. 32,33
- Periodontal regeneration: xHyA gel has demonstrated significant regenerative properties in various periodontal defects, including gingival recessions 34, infrabony defects 35, and furcations.36 It promotes the regeneration of periodontal ligament, cementum, and alveolar bone.
- Extended barrier effect: When combined with a collagen membrane, cross-linked hyaluronic acid (xHya) slows down its resorption, extending its barrier effect. This is particularly advantageous for patients with high collagenase metabolism, such as diabetics.37
- Scaffold and volume preservation: xHyA gel acts as a biocompatible liquid scaffold that gradually resorbs over time.38 It serves as a placeholder in se or in conjunction with other biomaterial (e.g. bone graft particles), filling the void between biomaterial particles, attracting and stabilizing the blood clot, while supporting cell migration, adherence and proliferation.39 This property helps to limit volume resorption, particularly relevant in implant dentistry.40,41,42
- Bacteriostatic and pocket treatment: When injected into periodontal and peri-implant pockets, cross-linked hyaluronic acid gel acts as a bacteriostatic shield,43 helping to reduce bacterial colonization. Additionally, it contributes to a significant reduction in pocket probing depth (PPD).44
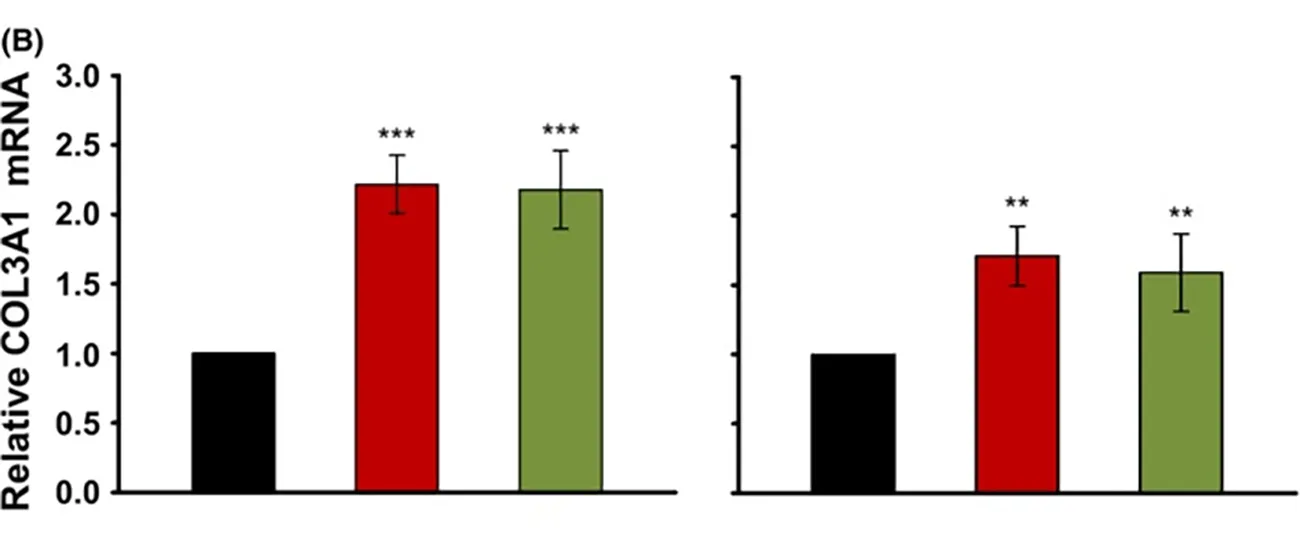
- Scarless wound healing: High molecular weight hyaluronic acid, whether native or slightly cross-linked, induces higher expression of collagen III (signals COL3A1, TGF-β3) , which is associated with scarless wound healing.45 This effect is observed in foetal wound healing, and can contribute to improved healing outcomes.
Conclusion: Reasons to use cross-linked hyaluronic acid gel in dental treatments
In summary, the use of combined native and cross-linked hyaluronic acid gel in dental treatments offers advantages such as prolonged presence, hydrophilic properties, enhanced cell attachment, reduced inflammation, periodontal regeneration, extended barrier effect (e.g. with collagen membranes), scaffold functionality, improved bone regeneration, bacteriostatic properties and scarless wound healing. By that, without changing the individual treatment protocol, an improved patient outcome can be achieved.
References
1. Meyer K, Palmer JW. The polysaccharide of the vitreous humor. Journal of Biological Chemistry. 1934;107(3):629-34.
2. Bartold PM, Page RC. Hyaluronic Acid Synthesized by Fibroblasts Cultured from Normal and Chronically Inflamed Human Gingivae. Collagen and Related Research. 1986;6(4):365-78.
3. Dahiya P, Kamal R. Hyaluronic Acid: A Boon in Periodontal Therapy. N Am J Med Sci. 2013;5(5):309-15.
4. Stern R, Asari AA, Sugahara KN. HAluronan fragments: An information-rich system. European Journal of Cell Biology. 2006;85(8):699-715
5. Husseini B, Friedmann A, Wak R, Ghosn N, Khoury G, El Ghoul T, Abboud CK, Younes R. Clinical and radiographic assessment of cross-linked hyaluronic acid addition in demineralized bovine bone based alveolar ridge preservation: A human randomized split-mouth pilot study. J Stomatol Oral Maxillofac Surg. 2023 Feb 16;124(4):101426. doi: 10.1016/j.jormas.2023.101426. Epub ahead of print. PMID: 36801259.
6. Kessiena L et al. ‘Hyaluronan in wound healing: Rediscovering a major player.’ Wound Rep Reg 2014;22:579-593.
7. Deed R et al. ‘Early response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan.’ Int J Cancer, 1997;71:51-56.
8. Asparuhova MB, Kiryak D, Eliezer M, Mihov D, Sculean A. Activity of two HAluronan preparations on primary human oral fibroblasts. J Periodontal Res. 2019 Feb;54(1):33-45. doi: 10.1111/jre.12602. Epub 2018 Sep 27. PMID: 30264516; PMCID: PMC6586051.
9. David-Raoudi M, Tranchepain F, Deschrevel B, et al. Differential effects of HAluronan and its fragments on fibroblasts: relation to wound healing. Wound Repair Regen. 2008;16:274‐287.
10. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with crosslinked hyaluronic acid-gel with or without a collagen matrix: a preclinical study in dogs. Quintessence International. 2021;52(4):308-16.
11. Bertl K, Gotfredsen K, Jensen SS, Bruckmann C, Stavropoulos A. Can hyaluronan injections augment deficient papillae at implant-supported crowns in the anterior maxilla? A randomized controlled clinical trial with 6 months follow-up. Clin Oral Implants Res. 2017 Sep;28(9):1054-1061. doi: 10.1111/clr.12917. Epub 2016 Jul 5. PMID: 27378556.
12. Romano, F., D’Anna, E., Aimetti, M., & Pugliese, M. (2019). A systematic review and meta-analysis of randomized controlled trials evaluating the efficacy of hyaluronic acid for interdental papilla reconstruction. Clinical Oral Investigations, 23(1), 1-10
13. Asparuhova MB, Kiryak D, Eliezer M, Mihov D, Sculean A. Activity of two HAluronan preparations on primary human oral fibroblasts. Journal of Periodontal Research. 2019;54(1):33-45.
14. Shirakata Y, Imafuji T, Nakamura T, Shinohara Y, Iwata M, Setoguchi F, Noguchi K, Sculean A. Crosslinked hyaluronic acid gel with or without a collagen matrix in the treatment of class III furcation defects: A histologic and histomorphometric study in dogs. J Clin Periodontol. 2022 Oct;49(10):1079-1089. doi: 10.1111/jcpe.13694. Epub 2022 Jul 21. PMID: 35817414; PMCID: PMC9796036.
15. Shirakata Y, Imafuji T, Nakamura T, Kawakami Y, Shinohara Y, Noguchi K, Pilloni A, Sculean A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with crosslinked hyaluronic acid-gel with or without a collagen matrix: a preclinical study in dogs. Quintessence Int. 2021;0(0):308-316. doi: 10.3290/j.qi.b937003. PMID: 33533237.
16. Shirakata Y, Nakamura T, Kawakami Y, Imafuji T, Shinohara Y, Noguchi K, Sculean A. Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a crosslinked hyaluronic acid gel. An experimental study in dogs. J Clin Periodontol. 2021 Apr;48(4):570-580. doi: 10.1111/jcpe.13433. Epub 2021 Feb 10. PMID: 33513277; PMCID: PMC8248173.
17. Pilloni A, Rojas MA, Marini L, Russo P, Shirakata Y, Sculean A, et al. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either Hyaluronic acid or an enamel matrix derivative: a 24-month randomized controlled clinical trial. Clin Oral Investig. 2021;25(8):5095-107.
18. Božić D, Ćatović I, Badovinac A, Musić L, Par M, Sculean A. Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials (Basel). 2021 Nov 11;14(22):6795. doi: 10.3390/ma14226795. PMID: 34832196; PMCID: PMC8624958.
19. Pilloni A, Rojas MA, Marini L, Russo P, Shirakata Y, Sculean A, et al. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either Hyaluronic acid or an enamel matrix derivative: a 24-month randomized controlled clinical trial. Clin Oral Investig. 2021;25(8):5095-107.
20. Eliezer M, Imber J, Radakovic S, Pirracchio L, Sculean A. The clinical effect of Hyaluronic acid on root recession coverage: a case series, 6 months evaluation. PD172, Poster Presentation, EuroPerio. 2018, Amsterdam.
21. Husseini B, Friedmann A, Wak R, Ghosn N, Khoury G, El Ghoul T, Abboud CK, Younes R. Clinical and radiographic assessment of crosslinked hyaluronic acid addition in demineralized bovine bone based alveolar ridge preservation: A human randomized split-mouth pilot study. J Stomatol Oral Maxillofac Surg. 2023 Feb 16;124(4):101426. doi: 10.1016/j.jormas.2023.101426. Epub ahead of print. PMID: 36801259.
22. Çankaya ZT, Gürbüz S, Bakirarar B, Kurtiş B. Evaluation of the Effect of Hyaluronic Acid Application on the Vascularization of Free Gingival Graft for Both Donor and Recipient Sites with Laser Doppler Flowmetry: A Randomized, Examiner-Blinded, Controlled Clinical Trial. Int J Periodontics Restorative Dent. 2020 Mar/Apr;40(2):233-243. doi: 10.11607/prd.4494. PMID: 32032408.
23. Asparuhova MB, Chappuis V, Stähli A, Buser D, Sculean A. Role of HAluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin Oral Investig. 2020 Nov;24(11):3923-3937. doi: 10.1007/s00784-020-03259-8. Epub 2020 Mar 31. PMID: 32236725; PMCID: PMC7544712.
24. Moseley R, Waddington RJ, Embery G. HAluronan and its potential role in periodontal healing. Dent Update. 2002 Apr;29(3):144-8. doi: 10.12968/denu.2002.29.3.144. PMID: 11989392.
25. Bayer IS. Hyaluronic Acid and Controlled Release: A Review. Molecules. 2020 Jun 6;25(11):2649. doi: 10.3390/molecules25112649. PMID: 32517278; PMCID: PMC7321085.
26. Husseini B, Friedmann A, Wak R, Ghosn N, Khoury G, El Ghoul T, Abboud CK, Younes R. Clinical and radiographic assessment of cross-linked hyaluronic acid addition in demineralized bovine bone based alveolar ridge preservation: A human randomized split-mouth pilot study. J Stomatol Oral Maxillofac Surg. 2023 Feb 16;124(4):101426. doi: 10.1016/j.jormas.2023.101426. Epub ahead of print. PMID: 36801259.
27. Nobis B, Ostermann T, Weiler J, Dittmar T, Friedmann A. Impact of Crosslinked hyaluronic Acid on Osteogenic Differentiation of SAOS-2 Cells in an Air-Lift Model. Materials (Basel). 2022 Sep 20;15(19):6528. doi: 10.3390/ma15196528. PMID: 36233870; PMCID: PMC9572243.
28. Stiller M. et al. ‘Performance of β-tricalcium phosphate granules and putty bone grafting materials after bilateral sinus floor augmentation in humans’ Biomaterials 2014
29. Alcantara CEP et al ‘Hyaluronic acid accelerates bone repair in human dental sockets: a randomized triple-blind clinical trial’ Braz Oral Res. 2018.DOI 10.1590/1807-3107bor-2018.vol32.0084
30. Mir-Mari, J, Wui, H, Jung, RE, Hämmerle, CHF, Benic, GI. Influence of blinded wound closure on the volume stability of different GBR materials: an in vitro cone-beam computed tomographic examination. Clin. Oral Impl. Res. 27, 2016, 258– 265. doi: 10.1111/clr.12590
31. Mueller A, Fujioka-Kobayashi M, Mueller HD, Lussi A, Sculean A, Schmidlin PR, Miron RJ. ‘Effect of Hyaluronic acid on morphological changes to dentin surfaces and subsequent effect on periodontal ligament cell survival, attachment, and spreading’ Clinical Oral Investigations 2016 May .DOI 10.1007/s00784-016-1856-6
32. Asparuhova M, Kiryak D, Eliezer M, Mihov D, Sculean A. ‘Activity of two HAluronan preparations on primary human oral fibroblasts’.J Periodontal Res 2018 Sep 27. Epub 2018 Sep 27
33. Yıldırım S, Özener HÖ, Doğan B, Kuru B. Effect of topically applied Hyaluronic acid on pain and palatal epithelial wound healing: An examiner-masked, randomized, controlled clinical trial. J Periodontol. 2018;89(1):36-45. doi:10.1902/jop.2017.170105
34. Shirakata Y, Nakamura T, Kawakami Y, Imafuji T, Shinohara Y, Noguchi K, Sculean A. Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a crosslinked hyaluronic acid gel. An experimental study in dogs. J Clin Periodontol. 2021 Apr;48(4):570-580. doi: 10.1111/jcpe.13433. Epub 2021 Feb 10. PMID: 33513277; PMCID: PMC8248173.
35. Shirakata Y, Imafuji T, Nakamura T, Kawakami Y, Shinohara Y, Noguchi K, Pilloni A, Sculean. A. Periodontal wound healing/regeneration of two-wall intrabony defects following reconstructive surgery with crosslinked hyaluronic acid-gel with or without a collagen matrix: a preclinical study in dogs. Quintessence Int. 2021;0(0):308-316. doi: 10.3290/j.qi.b937003. PMID: 33533237.
36. Shirakata Y, Nakamura T, Kawakami Y, Imafuji T, Shinohara Y, Noguchi K, Sculean A. Healing of buccal gingival recessions following treatment with coronally advanced flap alone or combined with a crosslinked hyaluronic acid gel. An experimental study in dogs. J Clin Periodontol. 2021 Apr;48(4):570-580. doi: 10.1111/jcpe.13433. Epub 2021 Feb 10. PMID: 33513277; PMCID: PMC8248173.
37. Eliezer M, Sculean A, Miron RJ, et al. ‘Hyaluronic acid slows down collagen membrane degradation in uncontrolled diabetic rats.’J Periodontal Res. 2019;00:1–9.
https://doi.org/10.1111/jre.12665
38. Eliezer M, Sculean A, Miron RJ, et al. ‘Hyaluronic acid slows down collagen membrane degradation in uncontrolled diabetic rats.’J Periodontal Res. 2019;00:1–9.
https://doi.org/10.1111/jre.12665
39. Asparuhova M, Kiryak D, Eliezer M, Mihov D, Sculean A. ‘Activity of two HAluronan preparations on primary human oral fibroblasts’.J Periodontal Res 2018 Sep 27. Epub 2018 Sep 27
40. Husseini B, Friedmann A, Wak R, Ghosn N, Khoury G, El Ghoul T, Abboud CK, Younes R. Clinical and radiographic assessment of cross-linked hyaluronic acid addition in demineralized bovine bone based alveolar ridge preservation: A human randomized split-mouth pilot study. J Stomatol Oral Maxillofac Surg. 2023 Feb 16;124(4):101426. doi: 10.1016/j.jormas.2023.101426. Epub ahead of print. PMID: 36801259.
41. Mir-Mari J, Wui H, Jung RE, H€ammerle CHF, Benic GI.Influence of blinded wound closure on the volume stability of different GBR materials: an in vitro cone-beam computed tomographic examination. Clin. Oral Impl. Res. 00, 2015, 1–8 doi: 10.1111/clr.12590
42. Kauffmann F et al (2023) unpublished
43. Pirnazar P, Wolinsky L, Nachnani S, Haake S, Pilloni A, Bernard GW. ‘Bacteriostatic effects of Hyaluronic acid.’ J Periodontol 1999;70:370–4.
44. Eliezer M, Imber JC, Sculean A, Pandis N, Teich S, ‘Hyaluronic acid as adjunctive to nonsurgical and surgical periodontal therapy: a systematic review and meta-analysis’, Clin O Inv
2019; doi: s00784-019-03012-w
45. Asparuhova MB, Kiryak D, Eliezer M, Mihov D, Sculean A. Activity of two HAluronan preparations on primary human oral fibroblasts. J Periodontal Res. 2019 Feb;54(1):33-45. doi: 10.1111/jre.12602. Epub 2018 Sep 27. PMID: 30264516; PMCID: PMC6586051.